Abstract
Purpose
Her-2 is an epidermal growth factor receptor expressed in some prostate cancers (PC) associated with outgrowth of the tumor. Dysregulation of some microRNAs is involved in the regulation of PC pathogenesis, whereas the role of miR-4319 in PC is unknown and addressed in the current study.
Methods
The levels of miR-4319 in PC tissues were determined by RT-qPCR and their association with patient survival was studied by Kaplan–Meier analysis. Targeted genes for miR-4319 were predicted by a bioinformatics algorithm and confirmed by a dual-luciferase reporter assay. Growth of cells of overexpression or inhibition of miR-4319 or Her-2 was analyzed by an MTT assay. Cell survival in response to a chemotherapeutic drug, estramustine (EM), was analyzed by CCK-8 assay. Cell apoptosis was evaluated by TUNEL assay and Western blotting for apoptosis-associated proteins.
Results
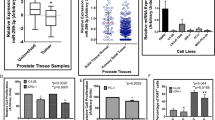
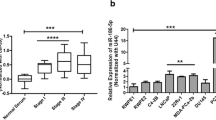
MiR-4319 levels were decreased in PC specimens, compared to corresponding normal prostate tissue. Lower levels of miR-4319 were correlated with poorer overall patients’ survival. In vitro, the cell survival mediated with Her-2 against chemotherapy was inhibited by overexpression of miR-4319 and was enhanced by depletion of miR-4319. Depletion of miR-4319 in primary prostate epithelial cells increased Her-2-dependent cell growth, while re-expression of miR-4319 in PC cells inhibited Her-2-dependent cell growth and Her-2-dependent resistance to EM-induced apoptosis.
Conclusion
The growth and chemo-resistance of PC cells may be suppressed via re-expression of miR-4319 that inhibits Her-2 signaling.






Similar content being viewed by others
References
Kruslin B, Ulamec M, Tomas D. Prostate cancer stroma: an important factor in cancer growth and progression. Bosn J Basic Med Sci. 2015;15(2):1–8.
Donkena KV, Yuan H, Young CY. Recent advances in understanding hormonal therapy resistant prostate cancer. Curr Cancer Drug Targets. 2010;10(4):402–10.
Seok H, Ham J, Jang ES, Chi SW. MicroRNA target recognition: insights from transcriptome-wide non-canonical interactions. Mol Cells. 2016;39(5):375–81.
Forman JJ, Legesse-Miller A, Coller HA. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proc Natl Acad Sci USA. 2008;105(39):14879–84.
Cao MX, Jiang YP, Tang YL, Liang XH. The crosstalk between lncRNA and microRNA in cancer metastasis: orchestrating the epithelial-mesenchymal plasticity. Oncotarget. 2017;8(7):12472–83.
Papagiannakopoulos T, Kosik KS. MicroRNA-124: micromanager of neurogenesis. Cell Stem Cell. 2009;4(5):375–6.
Ivey KN, Muth A, Arnold J, King FW, Yeh RF, Fish JE, et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell. 2008;2(3):219–29.
Li Q, Gregory RI. MicroRNA regulation of stem cell fate. Cell Stem Cell. 2008;2(3):195–6.
Albano F, Anelli L, Zagaria A, Coccaro N, Casieri P, Minervini A, et al. SETBP1 and miR_4319 dysregulation in primary myelofibrosis progression to acute myeloid leukemia. J Hematol Oncol. 2012;5:48.
Murray NP, Reyes E, Fuentealba C, Jacob O, Orellana N. Possible role of HER-2 in the progression of prostate cancer from primary tumor to androgen independence. Asian Pac J Cancer Prev. 2015;16(15):6615–9.
Le Page C, Koumakpayi IH, Peant B, Delvoye N, Saad F, Mes-Masson AM. ErbB2/Her-2 regulates the expression of Akt2 in prostate cancer cells. Prostate. 2012;72(7):777–88.
Ricciardelli C, Jackson MW, Choong CS, Stahl J, Marshall VR, Horsfall DJ, et al. Elevated levels of HER-2/neu and androgen receptor in clinically localized prostate cancer identifies metastatic potential. Prostate. 2008;68(8):830–8.
Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015; 4. https://doi.org/10.7554/eLife.05005.
Koie T, Mitsuzuka K, Yoneyama T, Narita S, Kawamura S, Kaiho Y, et al. Neoadjuvant luteinizing-hormone-releasing hormone agonist plus low-dose estramustine phosphate improves prostate-specific antigen-free survival in high-risk prostate cancer patients: a propensity score-matched analysis. Int J Clin Oncol. 2015;20(5):1018–25.
Taplin ME, Xie W, Bubley GJ, Ernstoff MS, Walsh W, Morganstern DE, et al. Docetaxel, estramustine, and 15-month androgen deprivation for men with prostate-specific antigen progression after definitive local therapy for prostate cancer. J Clin Oncol. 2006;24(34):5408–13.
Seidman AD, Scher HI, Petrylak D, Dershaw DD, Curley T. Estramustine and vinblastine: use of prostate specific antigen as a clinical trial end point for hormone refractory prostatic cancer. J Urol. 1992;147(3 Pt 2):931–4.
Hardwick JM, Chen YB, Jonas EA. Multipolar functions of BCL-2 proteins link energetics to apoptosis. Trends Cell Biol. 2012;22(6):318–28.
Chan A. Neratinib in HER-2-positive breast cancer: results to date and clinical usefulness. Ther Adv Med Oncol. 2016;8(5):339–50.
Agus DB, Akita RW, Fox WD, Lofgren JA, Higgins B, Maiese K, et al. A potential role for activated HER-2 in prostate cancer. Semin Oncol. 2000;27(6 Suppl 11):76–83 (discussion 92–100).
Jomrich G, Schoppmann SF. Targeting HER 2 and angiogenesis in gastric cancer. Expert Rev Anticancer Ther. 2016;16(1):111–22.
Baxevanis CN, Voutsas IF, Gritzapis AD, Perez SA, Papamichail M. HER-2/neu as a target for cancer vaccines. Immunotherapy. 2010;2(2):213–26.
Goto K, Ishikawa S, Honma R, Tanimoto K, Sakamoto N, Sentani K, et al. The transcribed-ultraconserved regions in prostate and gastric cancer: DNA hypermethylation and microRNA-associated regulation. Oncogene. 2016;35(27):3598–606.
Qin J, Ke J, Xu J, Wang F, Zhou Y, Jiang Y, et al. Downregulation of microRNA-132 by DNA hypermethylation is associated with cell invasion in colorectal cancer. OncoTargets Ther. 2015;8:3639–48.
Barros-Silva D, Costa-Pinheiro P, Duarte H, Sousa EJ, Evangelista AF, Graca I, et al. MicroRNA-27a-5p regulation by promoter methylation and MYC signaling in prostate carcinogenesis. Cell Death Dis. 2018;9(2):167.
Suh SO, Chen Y, Zaman MS, Hirata H, Yamamura S, Shahryari V, et al. MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis. 2011;32(5):772–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Research involving human participants
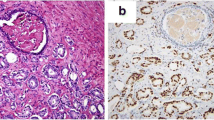
40 clinical samples and correspondingly normal tissues (NT) were resected postoperatively from PC patients (PC, stage III), during 2009 and 2011 from The Fourth Hospital of Harbin Medical University. All patients signed the written informed consent. All the patients were diagnosed with pathological and/or cytological examinations done by experienced pathologists based on the WHO classification criteria. Samples were collected before patients receiving chemotherapy or radiotherapy. All the samples were immediately saved at − 70 °C for further amplification. The follow-up lasted for 5 years for all the patients.
Informed consent
For the use of these clinical materials for research purposes, prior patient consents and approval from the Institutional Research Ethics Committee were obtained.
Rights and permissions
About this article
Cite this article
Lin, X., Wang, Y. Re-expression of microRNA-4319 inhibits growth of prostate cancer via Her-2 suppression. Clin Transl Oncol 20, 1400–1407 (2018). https://doi.org/10.1007/s12094-018-1871-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1871-y