Abstract
Purpose
Management of metastatic disease in oncology includes monitoring of therapy response principally by imaging techniques like CT scan. In addition to some limitations, the irruption of liquid biopsy and its application in personalized medicine has encouraged the development of more efficient technologies for prognosis and follow-up of patients in advanced disease.
Methods
PrediCTC constitutes a panel of genes for the assessment of circulating tumor cells (CTC) in metastatic colorectal cancer patients, with demonstrated improved efficiency compared to CT scan for the evaluation of early therapy response in a multicenter prospective study. In this work, we designed and developed a technology transfer strategy to define the market opportunity for an eventual implementation of PrediCTC in the clinical practice.
Results
This included the definition of the regulatory framework, the analysis of the regulatory roadmap needed for CE mark, a benchmarking study, the design of a product development strategy, a revision of intellectual property, a cost-effectiveness study and an expert panel consultation.
Conclusion
The definition and analysis of an appropriate technology transfer strategy and the correct balance among regulatory, financial and technical determinants are critical for the transformation of a promising technology into a viable technology, and for the decision of implementing liquid biopsy in the monitoring of therapy response in advanced disease.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
Sargent D, Sobrero A, Grothey A, O’Connell MJ, Buyse M, Andre T, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27(6):872–7.
Brandi G, De Lorenzo S, Nannini M, Curti S, Ottone M, Dall’Olio FG, et al. Adjuvant chemotherapy for resected colorectal cancer metastases: literature review and meta-analysis. World J Gastroenterol. 2016;22(2):519–33.
Van Cutsem E, Verheul HM, Flamen P, Rougier P, Beets-Tan R, Glynne-Jones R, et al. Imaging in colorectal cancer: progress and challenges for the clinicians. Cancers (Basel). 2016;8(9):81.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail GY, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20(7):1223–9.
Negin BP, Cohen SJ. Circulating tumor cells in colorectal cancer: past, present, and future challenges. Curr Treat Options Oncol. 2010;11(1–2):1–13.
Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail GY, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213–21.
Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59(1):110–8.
Barbazan J, Muinelo-Romay L, Vieito M, Candamio S, Diaz-Lopez A, Cano A, et al. A multimarker panel for circulating tumor cells detection predicts patient outcome and therapy response in metastatic colorectal cancer. Int J Cancer. 2014;135(11):2633–43.
Vidal Insua Y, Camara J, Vazquez EB, Fernandez A, Rivera F, Silva MJVV, et al. Predicting outcome and therapy response in mCRC patients using an indirect method for CTCs detection by a multigene expression panel: a multicentric prospective validation study. Int J Mol Sci. 2017;18(6):1265.
Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12(14):4218–24.
de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302–9.
Lankiewicz S, Zimmermann S, Hollmann C, Hillemann T, Greten TF. Circulating tumour cells as a predictive factor for response to systemic chemotherapy in patients with advanced colorectal cancer. Mol Oncol. 2008;2(4):349–55.
Anton C, Abal M, Alonso-Alconada L, Candamio S, Lopez-Lopez R, Martin-Saborido C. Monitoring treatment response in metastatic colorectal cancer: economic evaluation of PrediCTC® versus CT scan (submitted).
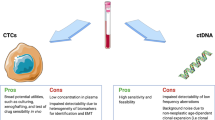
Tan CRC, Zhou L, El-Deiry WS. Circulating tumor cells versus circulating tumor dna in colorectal cancer: pros and cons. Curr Colorectal Cancer Rep. 2016;12(3):151–61.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial support
Pre-commercial Development of Research Results Program (PRIS), from the Galician Health System (SERGAS); “la Caixa” Banking Foundation call CaixaImpulse 2015.
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was signed by all patients.
Appendix: CLAIMS (PCT/EP2015/056649)
Appendix: CLAIMS (PCT/EP2015/056649)
-
1.
A method for determining the outcome of a subject suffering from metastatic colorectal cancer (mCRC) and/or the effectiveness of a therapy administered to said subject that comprises the steps of:
-
(a)
taking a first follow-up blood sample from the subject after having been administered at least one therapy cycle;
-
(b)
assessing the expression level of at least one gene selected from the set of genes of the group of LOXL3, ZEB2, GAPDH, VIL1, TIMP1, CLU and TLN1 in the CTCs of the blood sample;
-
(c)
classifying the expression level of the selected gene, or of each one of the set of genes selected for being analyzed, in the CTCs of the blood sample as “high” when it is higher than a reference cutoff value of the expression level of said gene and “low” when it is equal or lower than said reference cutoff value;
-
(d)
classifying the blood sample as
-
(i)
“low-CTC”, if the expression level of the selected gene in CTCs is low or, when more than one gene of the above mentioned set of genes is being analyzed, if the expression level in CTCs of more than a half of said analyzed genes is low;
-
(ii)
“high-CTC”, if the expression level of the selected gene in CTCs is high or, when more than one gene of the above mentioned set of genes is being analyzed, if the expression level in CTCs of at least one half of said analyzed genes is high;
-
(i)
-
(e)
assessing the effectiveness of the administered therapy by classifying the subject as:
-
(i)
“non-responder” to the therapy, if the subject is “high-CTC” for the analyzed follow-up blood sample, and
-
(ii)
“responder” to the therapy, if the subject is “low-CTC” for the analyzed follow-up blood sample;
-
(i)
-
(f)
optionally, taking a second or additional follow-up blood samples from the subject after having been administered a therapy cycle subsequent to the therapy cycle already administered when the first follow-up sample was taken and repeating steps (b) to (e) with said second or subsequent follow-up sample to confirm the previous assessment of therapy effectiveness;
-
(g)
additionally, or alternatively to steps (e) and (f), predicting the outcome of the subject at a time point of therapy course by classifying the subject as a “high risk” subject when the blood sample taken at that time point is “high-CTC”, and as a “low risk” subject when the blood sample taken at that time point is “low-CTC”.
-
(a)
-
2.
The method according to claim 1, wherein the expression level of at least two, three, four, five, six or all the seven genes of the group of LOXL3, ZEB2, GAPDH, VIL1, TIMP1, CLU and TLN1 is determined and classified in steps (a) and (b) and used for classifying the blood sample and the patients in step (c) and (d) and (e) and, when carried out, in step (e) and/or (f).
-
3.
The method according to claim 1 or 2, wherein LOXL3 and/or ZEB2 is selected for determining and classifying its expression level in steps (a) and (b) and used for classifying the blood sample and the patients in step (c) and (d) and, optionally, in step (e) and, when carried out, in step (e) and/or (f).
-
4.
The method according to claim 2 and 3, wherein the expression level of at least the two genes LOXL3 and VIL1 is determined and classified in steps (a) and (b) and used for classifying the blood sample and the patients in step (c) and (d) and, when carried out, in step (e) and/or (f).
-
5.
The method according to claim 4, wherein the expression level of at least the three genes LOXL3, VIL1 and CLU is determined and classified in steps (a) and (b) and used for classifying the blood sample and the patients in step (c) and (d) and, when carried out, in step (e) and/or (f).
-
6.
The method according to claim 5, wherein the expression level of at least the four genes LOXL3, VIL1, CLU and GAPDH is determined and classified in steps (a) and (b) and used for classifying the blood sample and the patients in step (c) and (d) and, when carried out, in step (e) and/or (f).
-
7.
The method according to any one of claims 1–3, wherein six genes of the group of GAPDH, VIL1, TIMP1, CLU, TLN1, LOXL3 and ZEB2 are selected for carrying out the steps of the method and the six genes are: GAPDH, VIL1, TIMP1, CLU, LOXL3 and ZEB2, or GAPDH, VIL1, CLU, TLN1, LOXL3 and ZEB2, or, GAPDH, VIL1, TIMP1, TLN1, LOXL3 and ZEB2, and wherein the blood sample to be classified in step (c) is classified as “low-CTC” when the expression level of at least four genes of the set of analyzed genes is low, and “high-CTC” when the expression level of three or more genes of the set of analyzed genes is high.
-
8.
The method according to any one of claims 1–3, wherein the seven genes of the group of GAPDH, VIL1, TIMP1, CLU, TLN1, LOXL3 and ZEB2 are selected for carrying out the steps of the method, and wherein the blood sample to be classified in step (c) is classified as “low-CTC” when the expression level of at least four genes of the set of analyzed genes is low, and “high-CTC” when the expression level of three or more genes of the set of analyzed genes is high.
-
9.
The method according to any one of claims 1–8, wherein a previous blood sample is taken from the subject before having administered the therapy cycle that has been administered when the first follow-up sample is taken, and steps (b)–(e) and, optionally, (f), and, optionally or alternatively to steps (e) and (f), step (g), are also performed in said previous blood sample.
-
10.
The method according to claim 9, wherein step (f) of confirmation is carried out when the subject is “low-CTC” for the follow-up blood sample considered in step (d) and “high-CTC” for the immediately previous sample.
-
11.
The method according to claim 10, wherein the follow-up sample considered in step (d) is the first follow-up blood sample and the immediately previous sample is the baseline sample taken before the start of the therapy.
-
12.
The method according to any one of claims 9–11, wherein step (g) is carried out for predicting the outcome of the patient before therapy, on a blood sample which is the baseline sample taken before the start of the therapy.
-
13.
The method according to any one of claims 1–11, wherein the administered therapy to be assessed is chemotherapy.
-
14.
The method according to claim 13, wherein the chemotherapy to be assessed comprised the administration of at least one fluoropyrimidine (fluorouracil or capecitabine) alone or in combination with oxaliplatin or irinotecan and/or with anti-EGFR or anti-VEGF antibodies.
-
15.
The method according to claim 13 or 14, wherein the first follow-up blood sample is taken before therapy cycle 2 or 4 weeks after the start of therapy.
-
16.
The method according to claim 14 or 15, wherein step (f) is carried out and a second follow-up blood sample is taken before therapy cycle 3 or 16 weeks after the start of therapy.
-
17.
The method according to any one of the preceding claims, wherein the expression level of each analyzed gene is normalized with regard to the expression level of a reference gene.
-
18.
The method according to claim 17, wherein the reference gene is CD45.
-
19.
The method according to any one of the preceding claims, wherein the blood samples are enriched in CTCs by using an immunoaffinity technique based on anti-EpCAM antibodies.
-
20.
The method according to any one of the preceding claims, wherein the expression level of each gene in each sample is assessed by quantifying the level of its corresponding mRNA in said sample.
-
21.
The method according to any one of the preceding claims, wherein each reference cutoff value that is used in step (c) has been determined in a statistical study with mCRC patients as the 75% percentile value of the expression level of the corresponding gene at the time point of the therapy course wherein the blood sample to be classified has been taken.
-
22.
The method according to any one of the preceding claims, wherein the assessment is complemented with the results of an imaging technique such a CT colonography and/or with a monitoring technique based on the determination of a biomarker in serum such as CA-125 or CEA.
Rights and permissions
About this article
Cite this article
Alonso-Alconada, L., Barbazan, J., Candamio, S. et al. PrediCTC, liquid biopsy in precision oncology: a technology transfer experience in the Spanish health system. Clin Transl Oncol 20, 630–638 (2018). https://doi.org/10.1007/s12094-017-1760-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1760-9