Abstract
Purpose
We conduct this study to compare the efficacy and toxicity of intensity-modulated radiotherapy (IMRT) concurrent weekly nedaplatin (NDP) versus IMRT alone in the stage III/IV non-surgical elderly patients with non-small-cell lung cancer (NSCLC).
Methods
117 patients were enrolled into our study. The patients were assigned into two different groups: radiotherapy (RT) group and chemoradiotherapy (CRT) group. Patients in RT group were treated with IMRT at a single daily dose of 2 Gy for 5 days per week, totally 52–66 Gy. The CRT group, IMRT concurrent weekly NDP at a dose of 25 mg/m2.
Results
In CRT group, the median survival was 11.0 months (95% confidence interval [CI], 8.894–13.106 months) and in RT group, it was 7.0 months (95% CI 5.771–8.229 months). The 1-year, 2-year, 3-year, survival rates in the combined treatment arm were higher than the radiation therapy arm (46.8 vs 25.9%, 25.1 vs 11.8%, 14.7 vs 8.0%; p < 0.001). The Cox’s multiple regression analysis showed that CRT had significantly better overall survival than RT (HR 0.523; 95.0% CI 0.338–0.807; p = 0.003). The objective response rate provided that 73.3% treated with CRT compared with 51.1% (p = 0.018) received RT alone. Of the hematologic toxicities, leukocytes (35.0 vs 0%; p < 0.001), neutrophils (33.3 vs 0%; p < 0.001) were significantly more common in the CRT group than the RT group.
Conclusions
We first discovered that NDP concurrent IMRT for treating stage III/IV non-surgical elderly patients with NSCLC was good curative effect of better objective response rate and well-tolerated. However, within the low number of patients, only stage IV gained a survival benefit.
Similar content being viewed by others
Introduction
Lung cancer is a major cause of tumor related mortality and the most common malignancy among people in most countries [1]. Of all cases of lung cancer, non-small-cell lung cancer (NSCLC) accounts for approximately 80% [2, 3]. There has been a significant increase in the occurring of NSCLC, with higher life expectancy worldwide, due to the relative accumulated increase in danger of tumor with age. The elderly patient is a special group regarded as 60 years or older in epidemiology according to their poorer body status, increased appearance of chronic disease complications, insensitive to therapy reaction [4]. Hurria and Siegel [5, 6] displayed that the incidence of NSCLC in elderly patients was high, in addition, the morbidity and mortality rates of patients-older than 60 years included 50% in patients-age range from 65 to 70 years and 30–40% of patients-older which than 70 years were higher. Except for patients who are susceptible to surgical resection, presently, most elderly NSCLC patients with locally or regionally advanced stage [7], at which the tumor is unresectable.
The past few years have seen the comprehensive implementation of intensity-modulated radiotherapy (IMRT) as one of the standard of care for cancer radiation therapy. The advent of IMRT enables dose distribution that is more conformal to tumoral well as gives permission to dose escalation to target volumes while sparing normal structures [8, 9]. Nevertheless, the appearance of distant metastases affects the prognosis of patients. A majority of studies provided that concurrent chemoradiotherapy (CCRT) for the older was limited by the physiological hypofunction, declined immune function, the higher hematology toxicity and gastrointestinal adverse reactions [10, 11].
Nedaplatin (NDP), synthesized by Shionogi & Co. Ltd. (Osaka, Japan) in 1983, is a second-generation platinum analog with decreased gastrointestinal and renal toxicities [12]. Unfortunately, since NDP is not commonly used throughout the world, there is lacking of reporters assessing the efficacy and toxicity of CCRT. Furthermore, most randomized clinical trials investigating optimal treatment of NSCLC exclude elderly patients, as a consequence of leaving practitioners having to depend on under-powered subset analysis or extrapolating data from younger cohorts who have various outcomes.
To find a valid and better tolerated therapeutic choice for elderly, especially who is unfit for operation, we first conduct this investigation to compare the efficacy and toxicity of radiotherapy (RT) concurrent weekly NDP versus RT alone in stage III/IV unresected elderly NSCLC.
Materials and methods
Patients
Between June 2012 and November 2016, a total of 117 patients with histologically or cytologically confirmed squamous or adenoid NSCLC in Department of Radiation Oncology of the Qianfoshan Hospital Affiliated Shandong University were retrospectively reviewed in this study. Written informed consent was obtained from all patients. This protocol was approved by the participating Institutional Review Board.
The eligibility criteria of patients who participated the trial included the following: (1) stage IIIA, IIIB, IV NSCLC (American Joint Committee on Cancer Staging Manual, ed 7); (2) an absolute neutrophil count greater than 1500/μL, a platelet count greater than 100,000/μL, a serum creatinine level less than 1.5 times the upper limit of normal (ULN), a forced expiratory volume in 1 s greater than 1 L, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, weight loss of <10% over the preceding 3 months who had complete medical records; (3) no previous chemotherapy, RT, no significant associated contraindications and surgical resection; (4) age range, 60–80 years; (5) measurable or assessable tumor; (6) no other malignancy.
Exclusion criteria included (1) acquiring anti-tumor therapy within 4 weeks; (2) other serious complications that prevent the patient completing the therapy.
Treatment
The standard of care for stage III NSCLC patients who are not appropriate surgical candidates is CCRT, especially definitive chemoradiation. As to the patients could not tolerate concurrent therapy, sequential chemoradiation or RT alone is appropriate for receiving. In regard to stage IV, RT is recommended for local palliation or prevention of symptoms included pain, bleeding, or obstruction. Systemic therapy is recommended for stage IV patients with extensive metastases. Palliative RT could be used for symptom relief as well prophylaxis at primary or distant sites in potential (NCCN Guidelines Version 3. 2017).
RT was provided by a linac accelerator. Patients in RT group were treated with IMRT at a single daily dose of 2 Gy for 5 days/week. The total dose was 52–66 Gy. In the chemoradiotherapy (CRT) group, NDP was administered at a dose of 25 mg/m2 once a week for 6 weeks, beginning on the first day of local RT. Treatment was begun when the patients meet the organ function criteria: neutrophil count ≥1500/μL, platelet count ≥75,000/μL, leukocyte count ≥3000/μL as well as less than grade 1 non-hematological toxicities, except alopecia. Physical tests, biochemical examination, a complete blood cell count and chest radiography were completed once a week. When meet disease progression or unacceptable toxicity, the treatment which was repeated every 6 weeks ended. The chemotherapy-associated toxicity was mainly included grade III and IV hematological toxicity and non-hematological toxicity.
Systemic therapy was given following RT/CRT with single agent, combination chemotherapy or tyrosine kinase inhibitor (TKI).
Evaluation of efficacy and toxicities
Efficacy determination referred to the RECIST 1.1 criteria. Complete response (CR) was regarded as the absolute disappearance of all target lesions for more than 4 weeks. Partial response (PR) was regarded as at least a 30% decrease in the aggregate of diameters of target lesions for more than one month, taking as reference the baseline sum diameters. Progressive disease (PD) was regarded as at least a 20% increase in the total of diameters of target lesions via taking as reference the smallest sum on research or the appearance of new lesions. Moreover, the sum ought to show an absolute increase of more than 5 mm. The rest of outcomes was performed as stable disease (SD). The objective response rate (ORR) was defined as (CR + PR). The clinical benefit rate (CBR) included CR, PR and SD. For every treatment cycle, a baseline computed tomography (CT) examination of the chest and a reassessment were provided for patients. During treatment with NDP, imaging examinations and hematologic were routinely performed. The safety end points included: (1) the incidence of treatment-related adverse events was based on National Cancer Institute Common Toxicity Criteria Version 3.0, (2) severe adverse events, and (3) laboratory abnormalities.
Statistical analyses
Statistical analyses were formed utilizing SPSS 23.0 software. The patient characteristics and incidences of toxicity between the CRT group and RT group were tested utilizing χ 2 test or Fisher exact test and Mann–Whitney rank-sum tests. The Common Toxicity Criteria (version 1) was used to grade toxicity. Survival rates were calculated from the initial time of RT using the Kaplan–Meier method, differences between the survival curves were determined using the Log-rank significance test, therapy was evaluated by Cox’s proportional hazard model. When diagnosis to death, survival was defined during the period. Two-tailed p-values <0.05 were considered statistically significant.
Results
Patient characteristics
Due to the early loss (6 in the CRT group), refusal (2 in the CRT group, 1 in the RT group) and PD (1 in the CRT group), 9 (9/69, 13.0%) patients in the CRT group and 1 (1/48, 2.0%) in the RT group of the 117 patients enrolled in the study were ineligible for completing the trial.
The characteristics of resulting in 107 patients are provided in Table 1. The two groups were well balanced with respect to age (p = 0.990), sex (p = 0.384), histology (p = 0.759), disease stage (p = 0.060), weight loss (p = 0.697), tumor laterality (p = 0.585) and systemic therapy (p = 0.268). The patients range from 60 to 80 years, with a median age of 68 years. The RT plus chemotherapy group (median age, 68 years) was similar to the IMRT alone (median age, 67 years). With regard to the thirty-two NSCLC patients who were treated with IMRT in stage IV, there were seven patients refusing systemic therapy and signing the formal consent; five patients were unable to receive systemic therapy with a poor condition after evaluated while twenty patients received systemic therapy after IMRT.
Tumor response
Of 60 patients in the CRT group, 12 patients completed CR (20%, p = 0.321), 32 showed PR (53.3%, p = 0.122), respectively. The ORR showed 73.3% (p = 0.018), the CBR was 85.0% (p = 0.011). Of 47 patients in the RT group, there were 6 cases of CR (12.8%, p = 0.321), 18 cases of PR (38.3%, p = 0.122), respectively. The ORR noted 51.1% (p = 0.018), the CBR was determined as 63.8% (p = 0.011) (Table 2). There was significant difference in rates of ORR and CBR between the treatment groups.
Patient survival
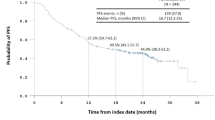
The median survival for those receiving chemotherapy plus IMRT was 11.0 months (95% confidence interval [CI], 8.894–13.106 months); those patients who received IMRT alone experienced a median survival of 7.0 months (95% CI 5.771–8.229 months). The 1-year, 2-year and 3-year survival rates in the combined treatment arm were 46.8, 25.1 and 14.7%, respectively; in the radiation therapy arm it was 25.9, 11.8 and 8.0%, respectively. The log-ranked statistical comparison indicated IMRT concurrent NDP resulted in a superior survival to p < 0.001 (Fig. 1). The cumulative hazard function is showed in Fig. 2. The result of univariate survival analysis provided that the CRT had notably better overall survival (OS) than the RT group (HR 0.486; 95.0% CI 0.316–0.746; p = 0.001). The Cox’s multiple regression analysis showed that the chemotherpy plus RT group had favorable prognosis outcome than the RT group (HR 0.523; 95.0% CI 0.338–0.807; p = 0.003).
In subgroup analysis, the Cox’s multiple regression analysis showed that CRT had significantly better OS than RT in stage IV (HR 0.494; 95.0% CI 0.284–0.858; p = 0.012). However, for stage III, the statistical comparison indicated no statistical significant superior survival between IMRT concurrent NDP and IMRT alone (HR 0.488; 95.0% CI 0.235–1.014; p = 0.055).
Toxicity
Most patients in each group (CRT and RT) were fully assessable for toxicity. Toxicity was evaluated by treatment group with Grade 3 and Grade 4 toxicity. The greater detail of the toxicities that occurred in ≥6 of 107 patients (5%) is summarized in Table 3. The patients who received combined treatment had a greater incidence of both severe hematologic and non-hematologic toxicity. Of the hematologic toxicities, leukocytes (36.7 vs 0%; p < 0.001), neutrophils (33.3 vs 0%; p < 0.001), thrombocytopenia (25.0 vs 0%; p < 0.001), serum glutamic transaminase (SGOT) (28.3 vs 2.1%; p < 0.001), serum glutamic pyruvic transaminase (SGPT) (26.7 vs 2.1%; p = 0.001) were obviously more common in patients who received IMRT concurrent NDP than IMRT alone. For the detail of the hematologic toxicities, except dyspnea, pneumonitis, oesophagitis, only nausea (25.0 vs 4.2%; p = 0.004) appeared more frequently in the CRT group rather than RT group. Separate from the total, 60 patients who were treated with CCRT approach present the adverse effects of advanced stage in Table 4. No case of treatment-related death.
Discussion
As a majority of patients with NSCLC are found at the advanced stage, with a pity, the majority have missed the opportunity of surgery. For patients who have no indications for surgery, RT, chemotherapy or chemo-radiation could be the possible cure. For one thing, initially, three separate studies performed that the best method to treat locally advanced NSCLC (LAD-NSCLC) was a comprehensive therapy-combined RT and chemotherapy which strengthened and complemented each other in a coordinated fashion [13,14,15]. What’s more, three other randomized prospective studies displayed a radio-sensitizing benefit to concurrent chemotherapy and radiation compared with radiation alone, with enhanced survival rates and improved local control later [16]. For another thing, CCRT resulted in better survival than sequential therapy [13]. It is worthwhile noting that these existing literature support that NSCLC patients could benefit from CCRT, however, concerns of excessive toxicity have likely hindered the practice. NDP is a second-generation anti-cancer platinum, which is considered having low gastrointestinal and renal toxicity [17]. Despite the findings of previous clinical trials, the considerations lack of detailed analysis of patients concerning the combined-modality of using NDP, especially for the elderly who is accepted as a special community. More specifically, there are limited data to explain the therapy of those elderly patients with stage III/IV NSCLC. Therefore, our research fills the gap above and is the first associated study-discovering a benefit survival of received NDP concurrent with IMRT for locally advanced elderly, more excited, appearing a well-tolerated reaction to toxicity.
Recently, the survival of patients with advanced NSCLC has provided a therapeutic challenge. In our present study, among the parameters, tumor disease and treatment strategies were shown to be independent predictive factors for OS. The median survival in CRT group was higher than RT group (11.0 months VS 7.0 months in the RT group), and CCRT could notably improve the 1-year, 2-year and 3-year survival rate in elderly patients with NSCLC compared to RT alone. These results were consistent with those of Ready and Vokes [18]. Interestingly, the Cox’s multiple regression analysis suggested that CRT had better OS than RT in stage IV, while no superior survival between IMRT concurrent NDP and IMRT alone in stage III. In this trial, it was observed that combined-modality therapy obtained a greater likelihood of improving survival for the stage IV elderly NSCLC. On the one hand, CCRT was thought to be the standard treatment modality for unresectable locally advanced stage III NSCLC. However, in the context of our observation, contrast with RT alone, CRT made no difference in survival for stage III. Digging into this problem we encountered, we found a phase III randomized trial in accordance with our results. By analyzing the overall survival time of LAD-NSCLC, contrast with RT alone, those investigators revealed that a concomitant chemoradiation therapy delivered survival benefits to stage IIIB patients, whereas similar in the stage IIIA patients [19]. As a possible explanation relating to the outcome in our date, it is conceivable meaningful in some instances-the proportion of stage IIIA and IIIB patients might work; moreover, a small number of patients could probably influence as well. Then a large population is also required for further studies to evaluate the efficacy of combined chemotherapy with NDP in unresected elderly stage III NSCLC.
Su et al. suggested that concurrent chemotherapy with aggressive thoracic radiotherapy (TRT) was shown to play an important role in improving OS for stage IV NSCLC [20]. Ouyang et al. pointed out that treatment of IV NSCLC undergoing concurrent chemotherapy and RT might prolong survival in a single-center prospective study [21]. These two investigations are supporting our results in some extent for stage IV survival of two therapies.
To the best of our knowledge, the side effects which CRT lead to are including hematologic and non-hematologic. As far as toxicity is concerned, leukopenia, neutropenia, thrombocytopenia, SGOT, SGPT belong to hematologic side effects. Nausea, vomiting, dyspnea, pneumonitis and oesophagitis belong to non-hematologic. In CRT group, grade 3 and 4 hematological toxicity were obtained, leukopenia and neutropenia were the most common toxicity, compared with RT group. The incidence of severe nausea was distinctly higher than RT group. The grade 3 and 4 pneumonitis, oesophagitis in CRT group were higher than RT group, but there were no noteworthy difference. In a word, it is feasible to speculate that the toxicities induced in chemoradio-therapeutic regimen are higher than IMRT alone.
In comparison with the publications below, the data with respect to haematological toxicities and non-haematological toxicities in our subgroup analysis suggest that IMRT with NDP might cause a certain number of severe radiation pneumonitis and esophagitis, as in this trial, might enable to reduce the grade of pneumonitis and esophagitis in advanced stage. Current recommendations include platinum- or taxane-based doublets with concurrent RT regimen that keep the lung cancer at bay for a time in most patients [22,23,24]. Sekine I and Nokihara H revealed that cisplatin, vinorelbine concurrent TRT before docetaxel consolidation therapy resulted in 81, 81, 10, 10% of patients associated with grade 3/4 leukopenia, neutropenia, pneumonitis, esophagitis in unresectable stage III NSCLC [25]. Gandara DR and Chansky K demonstrated that cisplatin and S-1 with concurrent TRT in unresectable stage III NSCLC patients undergone grade 3/4 leukopenia in 50%, neutropenia in 25%, pneumonitis in 26% of patients [26]. A Japanese study reported that cisplatin or carboplatin, paclitaxel and docetaxel with concurrent RT resulted in an incidence of 13% acute grade 3/4 pneumonitis toxicity and 13% grade 3/4 esophageal toxicity in stage III elderly NSCLC patients [27]. The consequences of our trial are considered remarkably lower toxicity than these three investigations.
A prospective single-center study approved that cisplatin in combination with docetaxel, paclitaxel, pemetrexed, or vinorelbine concurrently with thoracic radiation in unresectable stage IV NSCLC patients accepted a 37.9, 39.5, 4, 1.5% incidence of grade 3/4 leukopenia, neutropenia, pneumonitis along with esophagitis, proposing a notion that the toxicity of this combined modality therapy setting was slightly higher than our approach [28].
Most clinical studies have performed that NDP is effective in lung cancer, esophageal cancer, head and neck carcinoma, cervical carcinoma, ovarian carcinoma, bladder carcinoma, testicular carcinoma and other solid carcinomas [29]. Lu et al. designed a phase I study of 70 mg/m2 NDP and 500 mg/m2 of pemetrexed combined with thoracic IMRT for inoperable stage III lung adenocarcinoma [30]. The median OS was 30.0 months (95% CI 16.4–43.6 months) and 2-year OS was 44.0% (95% CI 18.7–69.2 months) with grade 3 or worse neutropenia (33.3%), pneumonitis (6.7%) and esophagitis (20.0%). NDP is considered administering a better synergistic effect when being used with other chemotherapy drugs, it might be the one reason to underline that those OS is significantly longer than our research. Additionally, the median age is younger than ours (62, range 48–68). Apart from this, the toxicity of esophagitis is more serious compare to the outcome of current study. Taking an overview of this phenomenon, frequency of delivery leading to severe toxicity is probably prescribed to explain it, as the dose of chemotherapy in this study is lower than before. Furthermore, in a phase II study, F Oshita and M Ohe conducted with NDP at 50 mg/m2 and irinotecan at 60 mg/m2, suggesting the median overall survivals was 36.0 months and experiencing grade 3/4 neutropenia in 32.8%, leukopenia in 38.8% along with no grade 3 pneumonitis or oesophagitis of patients (median age, 62 years; range, 43–69 years) for inoperable stage III NSCLC [17], which seems to be somewhat confirm our consideration. Yukihiro Hasegawa et al. performed a phase I/II study to recommend that when given weekly nedaplatin 20 mg/m2 and paclitaxel 35 mg/m2 with concomitant thoracic RT was safety and effective in stage III NSCLC patients (median age, 62; range, 43–69) [7]. The monitored dose of NDP was similar with our regimen. Based on these three previous clinical trials, however, the decisions lacking the associated assessment of elderly specifically at least 70 years old with advanced NSCLC and the age of patients were ranging from 43 to 69 years old. Of note, timing of research is important to establish, in view of this issue, we afford the opportunity for designing and accomplishing it.
Although the analysis provides the encouraging results, the current study includes some limitations. First, it is performed with retrospective methodology. Second, the number of patients is relatively small. Hopefully, a large sample population is needed to warrant cumulative estimates.
Conclusions
We first discovered that NDP concurrent IMRT for treating stage III/IV non-surgical elderly patients with NSCLC was good curative effect of better objective response rate and well-tolerated. However, within the low number of patients, only stage IV gained a survival benefit.
References
Pastorino U. Lung cancer screening. Br J Cancer. 2010;102(12):1681–6. doi:10.1038/sj.bjc.6605660.
Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153–6.
Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the surveillance, epidemiology, and end results (SEER) program. Oncologist. 2003;8(6):541–52.
Lilenbaum RC, Herndon JE 2nd, List MA, Desch C, Watson DM, Miller AA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(1):190–6. doi:10.1200/JCO.2005.07.172.
Hurria A, Kris MG. Management of lung cancer in older adults. CA Cancer J Clin. 2003;53(6):325–41.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi:10.3322/caac.21166.
Hasegawa Y, Takanashi S, Okudera K, Aoki M, Basaki K, Kondo H, et al. Weekly paclitaxel and nedaplatin with concurrent radiotherapy for locally advanced non-small-cell lung cancer: a phase I/II study. Jpn J Clin Oncol. 2004;34(11):647–53. doi:10.1093/jjco/hyh119.
MacDonald SM, Ahmad S, Kachris S, Vogds BJ, DeRouen M, Gittleman AE, et al. Intensity modulated radiation therapy versus three-dimensional conformal radiation therapy for the treatment of high grade glioma: a dosimetric comparison. J Appl Clin Med Phys. 2007;8(2):47–60.
Tsien CI, Brown D, Normolle D, Schipper M, Piert M, Junck L, et al. Concurrent temozolomide and dose-escalated intensity-modulated radiation therapy in newly diagnosed glioblastoma. Clin Cancer Res. 2012;18(1):273–9. doi:10.1158/1078-0432.CCR-11-2073.
Wang T, Zhang SF, Qiu MQ, Li QL. Efficacy and safety of S-1 (tegafur, gimeracil, and oteracil potassium) concurrent with 3-dimensional conformal radiotherapy for newly diagnosed squamous cell carcinoma of the lung in elderly patients. Cancer Radiother. 2016;20(3):181–6. doi:10.1016/j.canrad.2015.12.004.
Segawa Y, Kiura K, Takigawa N, Kamei H, Harita S, Hiraki S, et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin Oncol. 2010;28(20):3299–306. doi:10.1200/JCO.2009.24.7577.
Ota K. Nedaplatin. Gan to kagaku ryoho Cancer Chemother. 1996;23(3):379–87.
Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–90. doi:10.1200/JCO.2009.26.2543.
Le Chevalier T, Arriagada R, Quoix E, Ruffie P, Martin M, Tarayre M, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst. 1991;83(6):417–23.
Dillman RO, Seagren SL, Propert KJ, Guerra J, Eaton WL, Perry MC, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med. 1990;323(14):940–5. doi:10.1056/NEJM199010043231403.
Sause WT, Scott C, Taylor S, Johnson D, Livingston R, Komaki R, et al. Radiation therapy oncology group (RTOG) 88-08 and eastern cooperative oncology group (ECOG) 4588: preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J Natl Cancer Inst. 1995;87(3):198–205.
Oshita F, Ohe M, Honda T, Murakami S, Kondo T, Saito H, et al. Phase II study of nedaplatin and irinotecan with concurrent thoracic radiotherapy in patients with locally advanced non-small-cell lung cancer. Br J Cancer. 2010;103(9):1325–30. doi:10.1038/sj.bjc.6605875.
Ready NE, Vokes EE. Induction or consolidation systemic therapy in the multimodality treatment of unresectable locally advanced non-small cell lung cancer. Lung Cancer. 2003;42(Suppl 1):S65–9.
Kim TY, Yang SH, Lee SH, Park YS, Im YH, Kang WK, et al. A phase III randomized trial of combined chemoradiotherapy versus radiotherapy alone in locally advanced non-small-cell lung cancer. Am J Clin Oncol. 2002;25(3):238–43.
Su SF, Hu YX, Ouyang WW, Lu B, Ma Z, Li QS, et al. Overall survival and toxicities regarding thoracic three-dimensional radiotherapy with concurrent chemotherapy for stage IV non-small cell lung cancer: results of a prospective single-center study. BMC Cancer. 2013;13:474. doi:10.1186/1471-2407-13-474.
Ouyang WW, Su SF, Hu YX, Lu B, Ma Z, Li QS, et al. Radiation dose and survival of patients with stage IV non-small cell lung cancer undergoing concurrent chemotherapy and thoracic three- dimensional radiotherapy: reanalysis of the findings of a single-center prospective study. BMC Cancer. 2014;14:491. doi:10.1186/1471-2407-14-491.
Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S Jr, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004;22(2):330–53. doi:10.1200/JCO.2004.09.053.
Jett JR, Schild SE, Keith RL, Kesler KA. American College of Chest Physicians. Treatment of non-small cell lung cancer, stage IIIB: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):266S–76S. doi:10.1378/chest.07-1380.
Robinson LA, Ruckdeschel JC, Wagner H Jr, Stevens CW. American College of Chest Physicians. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):243S–65S. doi:10.1378/chest.07-1379.
Sekine I, Nokihara H, Sumi M, Saijo N, Nishiwaki Y, Ishikura S, et al. Docetaxel consolidation therapy following cisplatin, vinorelbine, and concurrent thoracic radiotherapy in patients with unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2006;1(8):810–5.
Gandara DR, Chansky K, Albain KS, Leigh BR, Gaspar LE, Lara PN Jr, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003;21(10):2004–10. doi:10.1200/JCO.2003.04.197.
Kim YH, Ahn SJ, Kim YC, Kim KS, Oh IJ, Ban HJ, et al. Predictive factors for survival and correlation to toxicity in advanced stage III non-small cell lung cancer patients with concurrent chemoradiation. Jpn J Clin Oncol. 2016;46(2):144–51. doi:10.1093/jjco/hyv174.
PDQ Screening and Prevention Editorial Board. Lung cancer screening (PDQ(R)): Health professional version. In: PDQ cancer information summaries. Accession number: 26389268. Bethesda: National Cancer Institute; 2002.
Ita M, Okafuji M, Fukuda K, Mitsuoka K, Hanakita T, Hayatsu Y. Concurrent chemoradiotherapy with new platinum compound nedaplatin in oral cancer. Oral Oncol. 2003;39(2):144–9.
Lu Y, Gu W, Deng J, Yang H, Yang W. A phase I study of nedaplatin, pemetrexed and thoracic intensity-modulated radiotherapy for inoperable stage III lung adenocarcinoma. BMC Cancer. 2016;16(1):775. doi:10.1186/s12885-016-2800-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the National Natural Science Foundation of China (Grant Number 81672974) and Youth Science Foundation of China (Grant Number 81602719).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chen, F., Hu, P., Liang, N. et al. Concurrent chemoradiotherapy with weekly nedaplatin versus radiotherapy alone in elderly patients with non-small-cell lung cancer. Clin Transl Oncol 20, 294–301 (2018). https://doi.org/10.1007/s12094-017-1716-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1716-0