Abstract
Purpose
To assess the efficacy and safety of liposomal cytarabine in the treatment of de novo and relapsed leptomeningeal involvement in children with primary CNS tumours.
Methods
Data from clinical charts were entered into a database for consecutive unselected patients (n=20) from nine Spanish centres. Diagnosis of leptomeningeal involvement was confirmed by cytology, MRI and/or CT scan. The dose of liposomal cytarabine used varied from 20 to 50 mg, by age.
Results
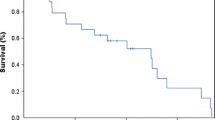
There were 8 females and 12 males, mean age 7.3 years (range 8 months to 18 years). The tumours were: 10 medulloblastomas, 4 ependymomas, 3 primitive neuroectodermal tumours and 3 other tumours. Fourteen had undergone previous chemotherapy and 12 radiotherapy. Nine received concurrent chemotherapy and 2 concurrent radiotherapy. Median follow-up was 244.5 days (range 12–869). Patients received a median of 5 doses (range 1–9) of liposomal cytarabine. A neurological response (complete or partial) was seen in 11/19 (58%) and a cytological response in 7/10 (64%). Median time to neurological progression exceeded 180 days (range 12–869). Adverse effects were reported in 11/20 patients, but none was grade IV.
Discussion
Liposomal cytarabine was well tolerated and efficacious in this patient group, but prospective randomised trials are needed.
Similar content being viewed by others
References
Baldwin RT, Preston-Martin S (2004) Epidemiology of brain tumors in childhood: a review. Toxicol Appl Pharmacol 199:118–131
Partap S, Fisher PG (2007) Update on new treatments and developments in childhood brain tumors. Curr Opin Pediatr 19:670–674
Chamberlain MC (1995) A review of leptomeningeal metastases in pediatrics. J Child Neurol 10:191–199
Kim S, Khatibi S, Howell SB et al (1993) Prolongation of drug exposure in cerebrospinal fluid by encapsulation into DepoFoam. Cancer Res 53:1596–1598
Chamberlain MC, Kormanik P, Howell SB et al (1995) Pharmacokinetics of intralumbar DTC-101 for the treatment of leptomeningeal metastases. Arch Neurol 52:912–917
Glantz MJ, LaFollette S, Jaeckle KA et al (1999) Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J Clin Oncol 17:3110–3116
Phuphanich S, Maria B, Braeckman R et al (2007) A pharmacokinetic study of intra-CSF adminis tered encapsulated cytarabine (DepoCyt) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma, or solid tumors as part of a phase III study. J Neurooncol 81:201–208
Angst MS, Drover DR (2006) Pharmacology of drugs formulated with DepoFoam: a sustained release drug delivery system for parenteral administration using multivesicular liposome technology. Clin Pharmacokinet 45:1153–1176
FDA. Depocyt Approval Letter. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21-041_DepoCyt_Approv.pdf. Accessed19 May 2011
EMEA. Depocyte European Public Assessment Report. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000317/WC500035644. pdf. Accessed 19 May 2011
Bomgaars L, Geyer JR, Franklin J et al (2004) Phase I trial of intrathecal liposomal cytarabine in children with neoplastic meningitis. J Clin Oncol 22:3916–3921
Slavc I, Peyrl A, Gupper A et al (2006) Tolerability of intrathecal liposomal cytarabine at a dose 25–50 mg in children 11 months to 15 years old. 7th Congress EANO in Vienna, Austria, September 14–17, 2006. Abstract 130. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1871663. Accessed 8 February 2008
Benesch M, Sovinz P, Krammer B et al (2007) Feasibility and toxicity of intrathecal liposomal cytarabine in 5 children and young adults with refractory neoplastic meningitis. J Pediatr Hematol Oncol 29:222–226
Björgvinsdóttir H, Heldrup J, Békássy A et al (2006) Intrathecal liposomal cytarabine: clinical experience in pediatric leukemia and neuroblastoma patients. Pediatr Blood Cancer 47[Suppl 4]:444
Peyrl A, Sauermann R, Slavc I et al (2008) Safety and pharmacokinetics of intrathecal liposomal cytarabine (DepoCyte®) in children <= 3 years of age with malignant brain tumors. Presented at: 13th ISPNO International Symposium on Pediatric Neuro-Oncology, Chicago, USA
Lassaletta A, Navajas A, Sabado C et al (2007) Intrathecal liposomal cytarabine in children with brain tumors. Presented at: 39th Annual Conference SIOP, Mumbai, India
Lassaletta A, Navajas A et al (2008) Intrathecal liposomal cytarabine in children with brain tumors. Presented at: SIOP 2008, 40th Annual Conference of International Society of Paediactric Oncology, Berlin, Germany
Lassaletta A, Perez-Olleros P, Scaglione C et al (2007) Successful treatment of intracranial ependymoma with leptomeningeal spread with systemic chemotherapy and intrathecal liposomal cytarabine in a two-year-old child. J Neurooncol 83:303–306
Napp Pharmaceuticals Limited (2009) Depo-Cyte 50 mg suspension for injection SPC. Available at: http://emc.medicines.org.uk/document.aspx?documentId=15047. Accessed 27 February 2009
Sommer C, Lackner H, Benesch M et al (2008) Neuroophthalmological side effects following intrathecal administration of liposomal cytarabine for central nervous system prophylaxis in three adolescents with acute myeloid leukaemia. Ann Hematol 87:887–890
Hospira. Cytarabine Injection Prescribing Information. Available at: http://www.hospira.com/_docs/Cytarabine%20483176-PROMOWEB.pdf.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Navajas, A., Lassaletta, Á., Morales, A. et al. Efficacy and safety of liposomal cytarabine in children with primary CNS tumours with leptomeningeal involvement. Clin Transl Oncol 14, 280–286 (2012). https://doi.org/10.1007/s12094-012-0796-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-012-0796-0