Abstract
Background
The aim of this study was to establish a new pathologic sub-classification of drug-induced liver injury (DILI) in combination with serum chemistry parameters and clinical observations.
Methods
From 777 DILI cases diagnosed in China–Japan Friendship Hospital from 2003 to 2014, 590 cases without other concomitant liver diseases were selected for the study. Pathological classification was established. Pathology and serum biochemical correlation analyses in 208 acute cases with complete biochemical data and prognostic information were conducted.
Results
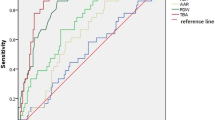
We established a pathological classification of DILI according to the target cells of the liver (hepatocytes, bile duct epithelial cells, liver vascular and sinusoidal endothelial cells). In the 590 cases of DILI analyzed, hepatocyte injury accounted for 67.0%, bile duct epithelial injury (including cholestasis and mixed type of injury) 23.9%, and vascular injury 8.8%; about half of them were caused by the administration of traditional Chinese herbal medicines. Acute hepatocyte injury (lobular hepatitis) is further divided into mild, moderate and severe subtypes, while the mixed type of injury is categorized as cholestatic hepatitis and mixed hepatitis. The dynamic liver enzyme curves were established between lobular hepatitis and mixed-type hepatitis based on the combined consideration of histopathology and serum chemistry data. We proved that R value > 5 with cholestasis is a special feature of mixed hepatitis, which clarified the suspicion of the previous clinical classification of R value. Greater attention should be paid to drug-induced bile duct vanishing syndrome and drug-induced vascular injury.
Conclusion
The pathological classification is simple to adopt and practically useful, which demonstrates the consistency between clinical features and liver pathology. The correlation between pathology and clinical biochemistry is an important way to acquire further understanding of DILI.







Similar content being viewed by others
References
Lee WM. Drug-induced hepatotoxicity. N Engl J Med 2003;349(5):474–485
Bjornsson ES. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin Liver Dis 2014;34(2):115–122
Teng GJ, Li BS, Zhao J, et al. Characteristics and trends of patients with drug-induced liver injury during the last ten years in China: a single-center experience. Chin Hepatol 2014;19(5):329–331
Shen T, Duan ZJ, Zhuang H. The epidemiology of drug-induced liver injury. Chin Hepatol 2015;20(10):19–23
China Food and Drug Administration. National annual report of drug induced adverse events in 2016. 2017;3:1–35. http://cdr-adr.org.cn/tzgg/ywgz/201705/W020170502330145305455.pdf
Hu XQ. Discussion on pathological scoring system of drug-induced liver injury. Chin J Hepatol 2012;20(3):176–177
Teschke R, Larrey D, Melchart D, Danan G. Traditional Chinese Medicine (TCM) and herbal hepatotoxicity: RUCAM and the role of novel diagnostic biomarkers such as microRNAs. Medicines 2016;3(3):E18
Danan G, Teschke R. Drug-induced liver injury: why is the Roussel Uclaf causality assessment method (RUCAM) still used 25 years after its launch? Drug Saf 2018;41(8):735–743
Branch of Infectious Diseases and Parasites of Chinese Medical Association, Branch of Liver of Chinese Medical Association. Prevention and treatment of viral hepatitis. Chin J Hepatol 2000;8(6):324–329
Popper H, Rubin E, Cardiol D, Schaffner F, Paronetto F. Drug-induced liver disease: a penalty for progress. Arch Intern Med 1965;115:128–136
Kleiner DE. The pathology of drug-induced liver injury. Semin Liver Dis 2009;29(4):364–372
Benichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol 1990;11(2):272–276
Zimmerman HJIK. Hepatic injury due to drugs and toxins. Pathology of the liver 4th ed. Edinburgh: Churchill Liveingstone; 2002. 621–710
Kleiner DE. The histopathological evaluation of drug-induced liver injury. Histopathology 2017;70(1):81–93
Robles-Diaz M, Lucena MI, Kaplowitz N, et al. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology 2014;147(1):109 e105–118 e105
Padda MS, Sanchez M, Akhtar AJ, Boyer JL. Drug-induced cholestasis. Hepatology 2011;53(4):1377–1387
Desmet VJ. Vanishing bile duct syndrome in drug-induced liver disease. J Hepatol 1997;26(Suppl 1):31–35
DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis 2002;22(1):27–42
Nakanuma Y, Tsuneyama K, Ohbu M, Katayanagi K. Pathology and pathogenesis of idiopathic portal hypertension with an emphasis on the liver. Pathol Res Pract 2001;197(2):65–76
Liu X, Wang TL, Xiang CH, et al. Liver pathology in idiopathic portal hypertension. Chin J Hepatol 2007;15(5):374–377
Gao H, Li N, Wang JY, et al. Definitive diagnosis of hepatic sinusoidal obstruction syndrome induced by pyrrolizidine alkaloids [Journal Article]. J Dig Dis 2012;13(1):33–39
Bjornsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: critical assessment based on published case reports. Hepatology 2016;63(2):590–603
Ren ZQ, Wang JH, Guo XY, et al. A review analysis of chinese literatures 2005–2014: clinical features of drug-induced liver injury. Chin J Pharmacoepidemiol 2016;0(5):284–289
Wang G-Q, Deng Y-Q, Hou F-Q. Overview of drug-induced liver injury in China. Clin Liver Dis 2014;4(1):26–29
Acknowledgements
We appreciate Dr. David E. Kleiner from the Laboratory of Pathology, National Cancer Institute, USA, who gave us very valuable suggestions and comments on this work before submission. We also thank Dr. Dalin (Dylan) Yao, a former US Food Drug Administration reviewer from JOINN Laboratories Inc., who helped us critically edit this paper.
Funding
This work was supported by grants from the National Natural Science Foundation of China (no. 81670545).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Tailing Wang, Xinyan Zhao, Chen Shao, Lihong Ye, Jing Guo, Na Peng, Honglei Zhang, Jian Li, Yuanyuan Kong, Hong You, Jidong Jia declare no conflict of interest.
Ethical approval
This article does not contain any study with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, T., Zhao, X., Shao, C. et al. A proposed pathologic sub-classification of drug-induced liver injury. Hepatol Int 13, 339–351 (2019). https://doi.org/10.1007/s12072-019-09940-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-019-09940-9