Abstract
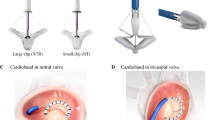
What is known about The Cardioband in the clinical area? Severe secondary mitral valve insufficiency frequently affects high-risk surgical patients. Cardioband system is a novel percutaneous surgical-like device for direct annuloplasty. It is totally implanted in the beating heart, by transvenous femoral access with minimal impact on hemodynamic and cardiac function during the implantation. It has been demonstrated to be safe and feasible in high-risk patients with functional mitral regurgitation, with high rates of implant success, significant annular reduction and regurgitation improvements. That positively impacts directly on ventricular function, functional status and quality of life. Recently, Cardioband has been used also in tricuspid position, with initial promising results.
Bibliographic review.




Similar content being viewed by others
References
Carpentier A. Cardiac valve surgery—the “French correction”. J Thorac Cardiovasc Surg. 1983;86:323–37.
De Bonis M, Maisano F, Canna GL, Alfieri O. Treatment and management of mitral regurgitation. Nat Rev Cardiol. 2011;9:133–46.
Detaint D, Sundt TM, Nkomo VT, Scott CG, Tajik AJ, Schaff HV, et al. Surgical correction of mitral regurgitation in the elderly: outcomes and recent improvements. Circulation. 2006;114:265–72.
Maisano F, Taramasso M, Nickenig G, et al. Cardioband, a transcatheter surgical-like direct mitral valve annuloplasty system: early results of the feasibility trial. Eur Heart J. 2016;37:817–25.
Testa L, Latib A, Montone RA, Bedogni F. Transcatheter mitral annuloplasty in chronic functional mitral regurgitation. 6-month results with the cardioband percutaneous mitral repair system.JACC Cardiovasc Interv. 2016;152:319–27.
Taramasso M, Guidotti A, Cesarovic N, et al. Transcatheter direct mitralannuloplasty with Cardioband: feasibility and efficacy trial in an acute preclinical model. EuroIntervention. 2016;12:e1428–34.
TRI-REPAIR: 30-Day Outcomes of Transcatheter TV Repair in Patients With Severe Secondary Tricuspid Regurgitation [Internet]. TCTMD.com. [cited 2017 Nov 12]. Available from: http://www.tctmd.com/slide/tri-repair-30-day-outcomes-transcatheter-tv-repair-patients-severe-secondary-tricuspid.
Edwards Cardioband Transcatheter Valve Repair System: expanding options for patients [Internet]. [cited 2018 Feb 8]. Available from: https://www.pcronline.com/Cases-resources-images/Resources/Course-videos-slides/2017/Edwards-Cardioband-Transcatheter-Valve-Repair-System-expanding-options-for-patients.
Maisano F, Buzzatti N, Taramasso M, Ottavio A. Mitral TranscatheterTechnologies. Rambam Maimonides Med J. 2013;4:e0015. Available from: http://www.rmmj.org.il/(S(rdwvey55d3hg32udqqav4qrb))/Pages/Article.aspx?manuId=270.
Wong VM, Wenk JF, Zhang Z, et al. The effect of mitral annuloplasty shape inischemic mitral regurgitation: a finite element simulation. Ann Thorac Surg. 2012;93:776–82.
Maisano F, Taramasso M. The Cardioband transcatheter direct mitral valve annuloplasty system. EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol. 2015;11:W58-59.
Maisano F, Vanermen H, Seeburger J, et al. DDirect access transcathetermitral annuloplasty with a sutureless and adjustable device: preclinical experience. Eur J Cardiothorac Surg. 2012;42:524–9.
Maisano F, Taramasso M, Guidotti A, Nietlispach F. The Cardioband: strategies for optimal patient selection and optimised results. EuroIntervention. 2016;12:Y61–3.
Maisano F, La Canna G, Latib A, et al. FFirst-in-man transseptal implantationof a “surgical-like” mitral valve annuloplasty device for functional mitral regurgitation. JACC Cardiovasc Interv. 2014;7:1326–8.
Nickenig G, Hammerstingl C, Schueler R, et al. Transcatheter mitralannuloplasty in chronic functional mitral regurgitation. 6-month results with the cardioband percutaneous mitral repair system. JACC Cardiovasc Interv. 2016;9:2039–47.
A. Vahanian. Multicentre trial results of the transcather mitral valve repair system for functional mitral regurgitation. In PCR London Valves 2017. Available from: https://www.pcronline.com/Courses/PCR-London-Valves/PCR-London-Valves-2017/Programme/Scientific-programme/Percutaneous-mitral-valve-repair-early-and-long-term-outcomes/Multicentre-trial-results-of-the-transcatheter-mitral-valve-repair-system-for-functional-mitral-regurgitation?auth=true.
Al-Hijji M, Fender EA, El Sabbagh A, Holmes DR. Current treatment strategies for tricuspid regurgitation. Curr Cardiol Rep. 2017;19:106. Available from: http://link.springer.com/10.1007/s11886-017-0920-4.
Vassileva CM, Shabosky J, Boley T, Markwell S, Hazelrigg S. Tricuspid valve surgery: The past 10 years from the Nationwide Inpatient Sample (NIS) database. J Thorac Cardiovasc Surg. 2012;143:1043–9.
TRI-REPAIR: TrIcuspid Regurgitation RePAIr With CaRdiobandTranscatheter System - Full Text View - ClinicalTrials.gov [Internet]. [cited 2018 Feb 2]. Available from: https://clinicaltrials.gov/ct2/show/NCT02981953.
Rave J. Valcare Medical Home [Internet]. Valcare Medical. [cited 2017 Nov 18]. Available from: http://www.valcaremedical.com/.
Ted F, Mayra G, Michael S. Emerging technologies for direct and indirect percutaneous mitral annuloplasty. Eurointervention J Eurointervention J [Internet]. 2016 Sep 18 [cited 2017 Nov 18];12(Supplement Y). Available from: https://www.pcronline.com/eurointervention/Y_issue/volume-12/supplement-y/23/emerging-technologies-for-direct-and-indirect-percutaneous-mitral-annuloplasty.html.
Hahn RT, Meduri CU, Davidson CJ, et al. Early feasibility study of a transcatheter tricuspid valve annuloplasty. J Am Coll Cardiol. 2017;69:1795–806.
Wan B, Rahnavardi M, Tian DH, et al. A meta-analysis of MitraClip system versus surgery for treatment of severe mitral regurgitation. Ann Cardiothorac Surg. 2013;2:683–92.
Kuwata S, Taramasso M, Guidotti A, Nietlispach F, Maisano F. Evaluation of Valtech’s transcatheter mitral valve repair device. Expert Rev Med Devices. 2017;14:189–95.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
F. Maisano is a consultant for St Jude Medical, Edwards Lifesciences, Abbott Vascular, Medtronic and Valtech and is the cofounder of and shareholder of 4Tech Cardio and receives royalties from Edwards Lifesciences. F. Nietlispach is a consultant for St Jude Medical, Medtronic and Edwards Lifesciences. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Not applicable
Appendices
Appendix 1. Patient’s selection for mitral annuloplasty with Cardioband
ACTIVE TRIAL: NCT03016975
Ages eligible for study: 18 years and older (adult, senior)
Sexes eligible for study: All.
Accepts healthy volunteers: No.
Inclusion criteria:
-
Age > 18 years
-
Clinically significant functional mitral regurgitation (MR)
-
NYHA class II-IV;
-
Patient hospitalized due to heart failure during 12 months prior to submission to Central Screening Committee OR elevated Brain Natriuretic Peptid (BNP)
-
Patient is able and willing to give informed consent and follow protocol procedures and comply with follow-up visit compliance
Exclusion criteria:
-
Degenerative MR including mixed degenerative/functional MR
-
Severe mitral annular calcification
-
Other severe valve disorders requiring intervention according to current ACC/AHA valve guidelines
-
MV anatomy which may preclude proper Edwards Cardioband deployment
-
Life expectancy of less than 12 months
-
Patient is participating in concomitant research studies of investigational products which have not reached their primary endpoint
-
Unwillingness or inability to undergo follow-up investigations/visits
-
Other medical, social, or psychological conditions that precludes appropriate consent and follow-up
REPAIR TRAIL NCT02703311
Ages eligible for study: 18 years and older (adult, senior)
Sexes eligible for study: All.
Accepts healthy volunteers: No.
Criteria.
Inclusion criteria:
-
Severe (3+ to 4+) secondary mitral regurgitation
-
Symptomatic heart failure (NYHA Class III-IVa) despite guideline-directed medical therapy including CRT if indicated
-
The Local Site Heart Team concur that surgery will not be offered as a treatment option and that medical therapy is the intended therapy.
-
Transfemoral access and transseptal deployment of the Cardioband is determined to be feasible
-
Subject is willing and able to provide informed consent and follow protocol
Exclusion criteria:
-
EF < 20%
-
LVEDD ≥ 70 mm
-
Heavily calcified annulus or leaflets
-
Significant CAD requiring revascularization
-
Active bacterial endocarditis
-
Any percutaneous coronary, carotid, endovascular intervention, or carotid surgery within 30 days or any coronary or endovascular surgery within 3 months
-
Renal insufficiency requiring dialysis
-
Life expectancy of less than 12 months
-
Subject is participating in concomitant research studies of investigational products that have not reached their primary endpoint
-
Pulmonary hypertension ≥ 70 mmHg at rest
-
MV anatomy which may preclude proper device treatment
-
Right-sided congestive heart failure with echocardiographic evidence of severe right ventricular dysfunction and/or severe tricuspid regurgitation
-
Severe liver disease
-
Patient is pregnant or lactating
-
Hypersensitivity to nickel or chromium
-
Clinically significant bleeding diathesis or coagulopathy
-
History of MV repair
-
TIA or CVA within 3 months
Appendix 2. Patient’s selection for tricuspid annuloplasty with Cardioband
TRIREAPIR TRIAL: NCT02981953
Ages eligible for study: 18 years and older (adult, senior)
Sexes eligible for study: All.
Accepts healthy volunteers: No.
Inclusion criteria:
-
Chronic functional tricuspid regurgitation (FTR) with annular diameter ≥ 40 mm with valve systolic pulmonary pressure (sPAP) ≤ 60 mmHg
-
New York Heart Association (NYHA) class II-IVa
-
Symptomatic despite guideline-directed medical therapy (GDMT); at minimum patient on diuretic regimen
-
LVEF ≥ 30%
-
Patient is willing and able to comply with all specified study evaluations
-
The Local Site Heart Team concur that surgery will not be offered as a treatment option
-
Transfemoral access of the Cardioband is determined to be feasible
Exclusion criteria:
-
Aortic, mitral, and/or pulmonic valve stenosis and/or regurgitation ≥ moderate
-
Severe uncontrolled hypertension (systolic BP ≥ 180 mmHg and/or diastolic BP ≥ 110 mmHg)
-
Previous TV repair or replacement
-
Presence of trans-tricuspid pacemaker or defibrillator leads which are determined as immobile or interfering in the rout of procedure, as evaluated by echocardiography
-
Active endocarditis
-
MI or known unstable angina within the 30 days prior to the index procedure
-
Any percutaneous coronary intervention (PCI) within 30 days prior to the index procedure or planned 3 months post the index procedure
-
Hemodynamic instability or on IV inotropes
-
Cerebrovascular accident (CVA) within the past 6 months
-
Subject is on chronic dialysis
-
Anemia (Hb < 9 g/L) not corrected by transfusion
-
Bleeding disorders or hypercoaguable state
-
Active peptic ulcer or active gastrointestinal (GI) bleeding
-
Contraindication to anticoagulants
-
Known allergy to stainless steel, nickel, and/or polyester
-
Pregnant or lactating, or female of childbearing potential with a positive pregnancy test 24 h before any study-related radiation exposure
-
In the judgment of the investigator, co-morbid condition(s) that could limit the subject’s ability to participate in the study, including compliance with follow-up requirements, or that could impact the scientific integrity of the study
-
Life expectancy of less than 12 months
-
Impaired judgment and/or is undergoing emergency treatment
-
Currently participating in another investigational drug or device study that has not completed the primary endpoint or that clinically interferes with the endpoints of this study
-
Intra-cardiac masses, thrombi, or vegetations
-
Patients with cardiac cachexia
Rights and permissions
About this article
Cite this article
Ferrero Guadagnoli, A., Taramasso, M., Saccocci, M. et al. Cardioband system: a novel percutaneous solution for atrioventricular valve insufficiency. Indian J Thorac Cardiovasc Surg 34 (Suppl 2), 133–143 (2018). https://doi.org/10.1007/s12055-018-0667-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-018-0667-6