Abstract
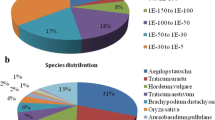
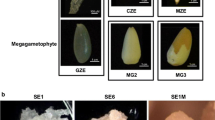
Somatic embryogenesis in mangosteen (Garcinia mangostana L.), an apomictic and recalcitrant-seeded species, allows stock improvement through genetic manipulation as well as mass propagation in a shortened time. To understand molecular events and gene expression during early somatic embryogenesis of mangosteen, we used RNA-Seq technology and assembled the mangosteen transcriptome de novo. Using the Trinity package, 186,203 transcripts were assembled with a mean size of 767 bp, 34.8 % of which showed significant similarities to known sequences in the GenBank non-redundant protein database. A total of 4001 transcripts were differentially expressed during somatic embryogenesis, while transcripts encoding signalling components of plant hormones such as auxin and cytokinin were significantly enriched. All the cytokinin-related genes were up-regulated, while some auxin-related genes were down-regulated and others were up-regulated. Somatic embryogenesis candidate genes such as SERK2, AGL15, CLVs, WUS and PIN1 were up-regulated in somatic embryos. Zinc finger and MADS family transcription factor genes were among the most presented differentially expressed transcripts in early somatic embryogenesis. Further qRT-PCR analysis confirmed the expression levels of the candidate genes. This study reports a de novo transcriptome assembly and analysis of gene expression during early somatic embryogenesis of mangosteen. Differentially expressed genes reveal that cytokinin has critical role in triggering somatic cell dedifferentiation and induction of somatic embryogenesis in mangosteen. The present work can serve as an important resource for further functional studies in woody plant embryogenesis.






Similar content being viewed by others
References
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. doi:10.1093/bioinformatics/btu170
Cabral GB, Carneiro VTC, Lacerda AL, do Valle CB, Martinelli AP, Dusi DMA (2011) Somatic embryogenesis and organogenesis in apomictic and sexual Brachiaria brizantha. Plant Cell Tiss Organ Cult 107:271–282. doi:10.1007/s11240-011-9978-7
Chen S-X, Wan M, Loh B-N (1996) Active constituents against HIV-1 protease from Garcinia mangostana. Planta Med 62:381–382. doi:10.1055/s-2006-957916
Chomnawang MT, Surassmo S, Nukoolkarn VS, Gritsanapan W (2007) Effect of Garcinia mangostana on inflammation caused by Propionibacterium acnes Fitoterapia 78:401–408 doi:http://dx.doi.org/10.1016/j.fitote.2007.02.019
Clark SE, Williams RW, Meyerowitz EM (1997) The CLAVATA1Gene encodes a putative receptor kinase that controls shoot and floral meristem size in arabidopsis. Cell 89:575–585. doi:10.1016/S0092-8674(00)80239-1
Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21:3674–3676. doi:10.1093/bioinformatics/bti610
Correia S, Cunha A, Salgueiro L, Canhoto J (2012) Somatic embryogenesis in tamarillo (Cyphomandra betacea): approaches to increase efficiency of embryo formation and plant development. Plant Cell Tiss Organ Cult 109:143–152. doi:10.1007/s11240-011-0082-9
Davies P (2010) The plant hormones: their nature, occurrence, and functions. In: Davies P (ed) Plant hormones. Springer, Netherlands, pp 1–15. doi:10.1007/978-1-4020-2686-7_1
De Rybel B et al (2013) A bHLH complex controls embryonic vascular tissue establishment and indeterminate growth in arabidopsis. Dev Cell 24:426–437. doi:10.1016/j.devcel.2012.12.013
Elbl P et al (2015) Comparative transcriptome analysis of early somatic embryo formation and seed development in Brazilian pine, Araucaria angustifolia (Bertol.). Kuntze Plant Cell Tiss Organ Cult 120:903–915. doi:10.1007/s11240-014-0523-3
Elhiti M, Tahir M, Gulden RH, Khamiss K, Stasolla C (2010) Modulation of embryo-forming capacity in culture through the expression of Brassica genes involved in the regulation of the shoot apical meristem. J Exp Bot 61:4069–4085. doi:10.1093/jxb/erq222
Elhiti M, Stasolla C, Wang A (2013) Molecular regulation of plant somatic embryogenesis. In Vitro Cell Dev Biol-Plant 49:631–642. doi:10.1007/s11627-013-9547-3
Elviana M, Rohani ER, Ismanizan I, Normah MN (2011) Morphological and histological changes during the somatic embryogenesis of mangosteen. Biol Plant 55:731–736. doi:10.1007/s10535-011-0177-5
Fehér A, Pasternak T, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Org Cult 74:201–228. doi:10.1023/A:1024033216561
Forestan C, Varotto S (2011) The role of PIN Auxin efflux carriers in polar Auxin transport and accumulation and their effect on shaping maize. Dev Molec Plant 5:787–798. doi:10.1093/mp/ssr103
Gaj M, Zhang S, Harada J, Lemaux P (2005) Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta 222:977–988. doi:10.1007/s00425-005-0041-y
Ge X-X, Chai L-J, Liu Z, Wu X-M, Deng X-X, Guo W-W (2012) Transcriptional profiling of genes involved in embryogenic, non-embryogenic calluses and somatic embryogenesis of Valencia sweet orange by SSH-based microarray. Planta 236:1107–1124. doi:10.1007/s00425-012-1661-7
Goebel-Tourand I, Mauro M-C, Sossountzov L, Miginiac E, Deloire A (1993) Arrest of somatic embryo development in grapevine: histological characterization and the effect of ABA, BAP and zeatin in stimulating plantlet development. Plant Cell, Tissue Org Cult 33:91–103. doi:10.1007/BF01997603
GOH HKL, RAO AN, LOH CS (1988) In Vitro Plantlet Formation in Mangosteen (Garcinia mangostana L.). Ann Bot 62:87–93
Goh C-J, Lakshmanan P, Loh C-S (1994) High frequency direct shoot bud regeneration from excised leaves of mangosteen (Garcinia mangostana L.). Plant Sci 101:173–180. doi:10.1016/0168-9452(94)90253-4
Grabherr MG et al (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotech 29:644–652, http://www.nature.com/nbt/journal/v29/n7/abs/nbt.1883.html-supplementary-information
Gray DJ, Purohit A, Triglano RN (1991) Somatic embryogenesis and development of synthetic seed technology. Crit Rev Plant Sci 10:33–61. doi:10.1080/07352689109382306
Gutierrez-Orozco F, Failla ML (2013) Biological activities and bioavailability of mangosteen xanthones: a critical review of the current evidence. Nutrients 5:3163–3183. doi:10.3390/nu5083163
Heck GR, Perry SE, Nichols KW, Fernandez DE (1995) AGL15, a MADS domain protein expressed in developing embryos. Plant Cell 7:1271–1282. doi:10.2307/3870101
Hou R et al (2011) Transcriptome sequencing and de novo analysis for Yesso Scallop (Patinopecten yessoensis) Using 454 GS FLX. PLoS ONE 6:e21560. doi:10.1371/journal.pone.0021560
Huang L-C, Huang B-L, Wang C, Kuo C-I, Murashige T (2000) Developing an improved In vitro propagation system for slow-growing species using garcinia mangostana L. (mangosteen). In Vitro Cell Dev Biol-Plant 36:501–504. doi:10.1007/s11627-000-0089-0
Huang C, Jia Y, Yang S, Chen B, Sun H, Shen F, Wang Y (2007) Characterization of ZNF23, a KRAB-containing protein that is downregulated in human cancers and inhibits cell cycle progression. Exp Cell Res 313:254–263. doi:10.1016/j.yexcr.2006.10.009
Hudson ME, Lisch DR, Quail PH (2003) The FHY3 and FAR1 genes encode transposase-related proteins involved in regulation of gene expression by the phytochrome A-signaling pathway. Plant J 34:453–471. doi:10.1046/j.1365-313X.2003.01741.x
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Org Cult 33:105–119. doi:10.1007/bf01983223
Hutchinson MJ, Saxena PK (1996) Role of purine metabolism in thidiazuron-induced somatic embryogenesis of geranium (Pelargonium × hortorum) hypocotyl cultures. Physiol Plant 98:517–522. doi:10.1111/j.1399-3054.1996.tb05706.x
Ipekci Z, Gozukirmizi N (2003) Direct somatic embryogenesis and synthetic seed production from Paulownia elongata. Plant Cell Rep 22:16–24. doi:10.1007/s00299-003-0650-5
Iraqi D, Tremblay FM (2001) Analysis of carbohydrate metabolism enzymes and cellular contents of sugars and proteins during spruce somatic embryogenesis suggests a regulatory role of exogenous sucrose in embryo development. J Exp Bot 52:2301–2311. doi:10.1093/jexbot/52.365.2301
Jin F et al (2014a) Comparative transcriptome analysis between somatic embryos (SEs) and zygotic embryos in cotton: evidence for stress response functions in SE development. Plant Biotechnol J 12:161–173. doi:10.1111/pbi.12123
Jin J, Zhang H, Kong L, Gao G, Luo J (2014b) PlantTFDB 3.0: a portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res 42:D1182–D1187. doi:10.1093/nar/gkt1016
Kanehisa M, Goto S (2000) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30. doi:10.1093/nar/28.1.27
Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M (2014) Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res 42:D199–D205. doi:10.1093/nar/gkt1076
Kunieda T et al (2008) NAC family proteins NARS1/NAC2 and NARS2/NAM in the outer integument regulate embryogenesis in arabidopsis. Plant Cell 20:2631–2642. doi:10.1105/tpc.108.060160
Lagacé M, Matton D (2004) Characterization of a WRKY transcription factor expressed in late torpedo-stage embryos of Solanum chacoense. Planta 219:185–189. doi:10.1007/s00425-004-1253-2
Leibfried A et al (2005) WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438:1172–1175, http://www.nature.com/nature/journal/v438/n7071/suppinfo/nature04270_S1.html
Lenhard M, Bohnert A, Jürgens G, Laux T (2001) Termination of stem cell maintenance in arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 105:805–814. doi:10.1016/S0092-8674(01)00390-7
Li B, Dewey C (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform 12; 323 doi:citeulike-article-id:9608780. doi: 10.1186/1471-2105-12-323
Li Z, Thomas TL (1998) PEI1, an embryo-specific zinc finger protein gene required for heart-stage embryo formation in Arabidopsis. Plant Cell 10:383–398
Lim AL (1984) The embryology of Garcinia Mangostana L. (Clusiaceae). U.S. Government Printing Office
Lin R, Ding L, Casola C, Ripoll DR, Feschotte C, Wang H (2007) Transposase-derived transcription factors regulate light signaling in arabidopsis. Science 318:1302–1305. doi:10.1126/science.1146281
Liu X et al (2011) AGAMOUS terminates floral stem cell maintenance in arabidopsis by directly repressing WUSCHEL through recruitment of polycomb group proteins. Plant Cell 23:3654–3670. doi:10.1105/tpc.111.091538
Lu X, Kim H, Zhong S, Chen H, Hu Z, Zhou B (2014) De novo transcriptome assembly for rudimentary leaves in Litchi chinesis Sonn. and identification of differentially expressed genes in response to reactive oxygen species. BMC Genomics 15:1–14. doi:10.1186/1471-2164-15-805
Maere S, Heymans K, Kuiper M (2005) BiNGO: a Cytoscape plugin to assess overrepresentation of Gene Ontology categories in biological networks. Bioinformatics 21:3448–3449. doi:10.1093/bioinformatics/bti551
Mahdavi-Darvari F, Noor N, Ismanizan I (2015) Epigenetic regulation and gene markers as signals of early somatic embryogenesis. Plant Cell Tiss Organ Cult 120:407–422. doi:10.1007/s11240-014-0615-0
Malik MR, Wang F, Dirpaul JM, Zhou N, Polowick PL, Ferrie AMR, Krochko JE (2007) Transcript profiling and identification of molecular markers for early microspore embryogenesis in Brassica napus. Plant Physiol 144:134–154. doi:10.1104/pp.106.092932
Mari S, Engelmannl F, Chabrillangel N, Huet C, Michaux-Ferrière N (1995) Histo-cytological study of apices of coffee (Coffea racemosa and C. sessiliflora) in vitro plantlets during their cryopreservation using the encapsulation-dehydration technique. Cryo-Letters 16:289–298
Marioni JC, Mason CE, Mane SM, Stephens M, Gilad Y (2008) RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res 18:1509–1517. doi:10.1101/gr.079558.108
Mayer KFX, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T (1998) Role of WUSCHEL in regulating stem cell fate in the arabidopsis shoot meristem. Cell 95:805–815. doi:10.1016/S0092-8674(00)81703-1
Metzker ML (2010) Sequencing technologies [mdash] the next generation. Nat Rev Genet 11:31–46
Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpan N, Neungton N (2004) Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J Ethnopharmacol 90:161–166. doi:10.1016/j.jep.2003.09.048
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Murray SC, Eckhoff P, Wood L, Paterson AH (2013) A proposal to use gamete cycling in vitro to improve crops and livestock. Nat Biotech 31:877–880. doi:10.1038/nbt.2707, http://www.nature.com/nbt/journal/v31/n10/abs/nbt.2707.html-supplementary-information
Murthy BNS, Murch SJ, Saxena PK (1995) Thidiazuron-induced somatic embryogenesis in intact seedlings of peanut (Arachis hypogaea): endogenous growth regulator levels and significance of cotyledons. Physiol Plant 94:268–276. doi:10.1111/j.1399-3054.1995.tb05311.x
Normah MN, Nor-Azza AB, Aliudin R (1995) Factors affecting in vitro shoot proliferation and ex vitro establishment of mangosteen. Plant Cell Tissue Org Cult 43:291–294. doi:10.1007/BF00039958
Ouyang X et al (2011) Genome-wide binding site analysis of FAR-RED ELONGATED HYPOCOTYL3 reveals its novel function in arabidopsis development. Plant Cell 23:2514–2535. doi:10.1105/tpc.111.085126
Park S, Harada J (2008) Arabidopsis embryogenesis. In: Suárez M, Bozhkov P (eds) Plant embryogenesis, vol 427. Methods in molecular biology™. Humana Press, pp 3–16. doi:10.1007/978-1-59745-273-1_1
Pérez-Rodríguez P, Riaño-Pachón DM, Corrêa LGG, Rensing SA, Kersten B, Mueller-Roeber B (2010) PlnTFDB: updated content and new features of the plant transcription factor database. Nucleic Acids Res 38:D822–D827. doi:10.1093/nar/gkp805
Perry SE, Lehti MD, Fernandez DE (1999) The MADS-domain protein AGAMOUS-like 15 accumulates in embryonic tissues with diverse origins. Plant Physiol 120:121–130
Petrášek J, Friml J (2009) Auxin transport routes in plant development. Development 136:2675–2688. doi:10.1242/dev.030353
Quiroz-Figueroa F, Méndez-Zeel M, Sánchez-Teyer F, Rojas-Herrera R, Loyola-Vargas VM (2002) Differential gene expression in embryogenic and non-embryogenic clusters from cell suspension cultures ofCoffea arabica. J Plant Physiol 159:1267–1270. doi:10.1078/0176-1617-00878
Quiroz-Figueroa F, Rojas-Herrera R, Galaz-Avalos R, Loyola-Vargas V (2006) Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tissue Org Cult 86:285–301. doi:10.1007/s11240-006-9139-6
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. doi:10.1093/bioinformatics/btp616
Rocha D, Monte-Bello C, Dornelas M (2015) Alternative induction of de novo shoot organogenesis or somatic embryogenesis from in vitro cultures of mature zygotic embryos of passion fruit (Passiflora edulis Sims) is modulated by the ratio between auxin and cytokinin in the medium. Plant Cell Tiss Organ Cult 120:1087–1098. doi:10.1007/s11240-014-0663-5
Rohani E, Ismanizan I, Noor N (2012) Somatic embryogenesis of mangosteen. Plant Cell Tiss Organ Cult 110:251–259. doi:10.1007/s11240-012-0147-4
Rounsley SD, Ditta GS, Yanofsky MF (1995) Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 7:1259–1269. doi:10.1105/tpc.7.8.1259
Salvo SAGD, Hirsch CN, Buell CR, Kaeppler SM, Kaeppler HF (2014) Whole transcriptome profiling of maize during early somatic embryogenesis reveals altered expression of stress factors and embryogenesis-related genes. PLoS ONE 9:e111407. doi:10.1371/journal.pone.0111407
Schellenbaum P, Jacques A, Maillot P, Bertsch C, Mazet F, Farine S, Walter B (2008) Characterization of VvSERK1, VvSERK2, VvSERK3 and VvL1L genes and their expression during somatic embryogenesis of grapevine (Vitis vinifera L.). Plant Cell Rep 27:1799–1809. doi:10.1007/s00299-008-0588-8
Schlereth A et al (2010) MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464:913–916, http://www.nature.com/nature/journal/v464/n7290/suppinfo/nature08836_S1.html
Schmidt EL, de Jong A, de Vries S (1994) Signal molecules involved in plant embryogenesis. In: Palme K (ed) Signals and signal transduction pathways in plants. Springer, Netherlands, pp 69–77. doi:10.1007/978-94-011-0239-1_4
Schmidt ED, Guzzo F, Toonen MA, de Vries SC (1997) A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124:2049–2062
Schoof H, Lenhard M, Haecker A, Mayer KFX, Jürgens G, Laux T (2000) The stem cell population of arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL. Genes Cell 100:635–644. doi:10.1016/S0092-8674(00)80700-X
Shi C-Y et al (2011) Deep sequencing of the Camellia sinensis transcriptome revealed candidate genes for major metabolic pathways of tea-specific compounds. BMC Genomics 12:131
Siriwardana S, Nabors MW (1983) Tryptophan enhancement of somatic embryogenesis in rice. Plant Physiol 73:142–146
Smith PM, Atkins CA (2002) Purine biosynthesis. big in cell division, even bigger in nitrogen assimilation. Plant Physiol 128:793–802
Srivastava LM (2002) Chapter 3 - embryogenesis. In: Srivastava LM (ed) Plant growth and development. Academic, San Diego, pp 75–92. doi:10.1016/B978-012660570-9/50143-X
Strickland SG, Nichol JW, McCall CM, Stuart DA (1987) Effect of carbohydrate source on alfalfa somatic embryogenesis. Plant Sci 48:113–121
Su YH, Zhang XS (2009) Auxin gradients trigger de novo formation of stem cells during somatic embryogenesis. Plant Signal Behav 4:574–576
Su YH, Zhao XY, Liu YB, Zhang CL, O’Neill SD, Zhang XS (2009) Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J 59:448–460. doi:10.1111/j.1365-313X.2009.03880.x
Suksamrarn S, Komutiban O, Ratananukul P, Chimnoi N, Lartpornmatulee N, Suksamrarn A (2006) Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem Pharm Bull 54:301–305. doi:10.1248/cpb.54.301
Sun L et al (2012) Differential gene expression during somatic embryogenesis in the maize (Zea mays L.) inbred line H99. Plant Cell Tiss Organ Cult 109:271–286. doi:10.1007/s11240-011-0093-6
Thakare D, Tang W, Hill K, Perry SE (2008) The MADS-domain transcriptional regulator AGAMOUS-LIKE15 promotes somatic embryo development in arabidopsis and soybean. Plant Physiol 146:1663–1672. doi:10.1104/pp.108.115832
Victor JMR, Murthy BNS, Murch SJ, KrishnaRaj S, Saxena PK (1999) Role of endogenous purine metabolism in thidiazuron-induced somatic embryogenesis of peanut (Arachis hypogaea L.). Plant Growth Regul 28:41–47. doi:10.1023/a:1006251531319
Viktor Nørgaard J, Krogstrup P (1991) Cytokinin induced somatic embryogenesis from immature embryos of Abies nordmanniana Lk. Plant Cell Rep 9:509–513. doi:10.1007/BF00232107
Vondráková Z, Eliášová K, Fischerová L, Vágner M (2011) The role of auxins in somatic embryogenesis of Abies alba. Centeurjbiol 6:587–596. doi:10.2478/s11535-011-0035-7
Wang X, Niu Q-W, Teng C, Li C, Mu J, Chua N-H, Zuo J (2009a) Overexpression of PGA37/MYB118 and MYB115 promotes vegetative-to-embryonic transition in Arabidopsis. Cell Res 19:224–235. http://www.nature.com/cr/journal/v19/n2/suppinfo/cr2008276s1.html
Wang Z, Gerstein M, Snyder M (2009b) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63. doi:10.1038/nrg2484
Wang JJ, Shi QH, Zhang W, Sanderson BJS (2012) Anti-skin cancer properties of phenolic-rich extract from the pericarp of mangosteen (Garcinia mangostana Linn.). Food Chem Toxicol 50:3004–3013. doi:10.1016/j.fct.2012.06.003
Weigel D, Jurgens G (2002) Stem cells that make stems. Nature 415:751–754
Willemsen V, Scheres B (2004) Mechanisms of pattern formation in plant embryogenesis. Annu Rev Genet 38:587–614. doi:10.1146/annurev.genet.38.072902.092231
Winter H, Huber SC (2000) Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit Rev Biochem Mol Biol 35:253–289. doi:10.1080/10409230008984165
Xie C et al (2011) KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res 39:W316–W322. doi:10.1093/nar/gkr483
Xu R, Quinn Li Q (2003) A RING-H2 zinc-finger protein gene RIE1 is essential for seed development in Arabidopsis. Plant Mol Biol 53:37–50. doi:10.1023/B:PLAN.0000009256.01620.a6
Xu Z et al (2013) Transcriptome profiling reveals auxin and cytokinin regulating somatic embryogenesis in different sister lines of cotton cultivar CCRI24. J Integr Plant Biol 55:631–642. doi:10.1111/jipb.12073
Yang X, Zhang X (2010) Regulation of somatic embryogenesis in higher plants. Crit Rev Plant Sci 29:36–57. doi:10.1080/07352680903436291
Yang X, Zhang X, Yuan D, Jin F, Zhang Y, Xu J (2012) Transcript profiling reveals complex auxin signalling pathway and transcription regulation involved in dedifferentiation and redifferentiation during somatic embryogenesis in cotton. BMC Plant Biol 12:1–19. doi:10.1186/1471-2229-12-110
Ye J et al (2006) WEGO: a web tool for plotting GO annotations. Nucleic Acids Res 34:W293–W297. doi:10.1093/nar/gkl031
Zeng F, Zhang X, Zhu L, Tu L, Guo X, Nie Y (2006) Isolation and characterization of genes associated to cotton somatic embryogenesis by suppression subtractive hybridization and macroarray. Plant Mol Biol 60:167–183. doi:10.1007/s11103-005-3381-x
Zhang Y, Zhang S, Han S, Li X, Qi L (2012) Transcriptome profiling and in silico analysis of somatic embryos in Japanese larch (Larix leptolepis). Plant Cell Rep 31:1637–1657. doi:10.1007/s00299-012-1277-1
Zheng W et al (2014) AtWuschel promotes formation of the embryogenic callus in Gossypium hirsutum. PLoS ONE 9:e87502. doi:10.1371/journal.pone.0087502
Acknowledgments
This research was supported by Science Fund 02-01-02-SF0847 under the Ministry of Science, Technology and Innovation (MOSTI), and a Malaysia and Research University Grant under the Arus Perdana (AP-2012-018) from the Universiti Kebangsaan Malasyia. The authors thank the Malaysia Genome Institute, in particular Mr. Mohd Faizal Abu Bakar for his support during the bioinformatics analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Jianguo Li
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
(XLSX 46.7 kb)
Supplementary Material 2
(XLSX 37.4 kb)
Supplementary Material 3
(JPG 57.3 kb)
Supplementary Material 4
(XLSX 1.13 mb)
Supplementary Material 5
(XLSX 48.9 kb)
Supplementary Material 6
(XLSX 94.4 kb)
Supplementary Material 7
(XLSX 58.7 kb)
Supplementary Material 8
(XLSX 16.6 mb)
Supplementary Material 9
(XLSX 50.5 kb)
Supplementary Material 10
(JPG 395 kb)
Rights and permissions
About this article
Cite this article
Mahdavi-Darvari, F., Noor, N.M. New Insight Into Early Somatic Embryogenesis of Mangosteen (Garcinia mangostana) Through de Novo and Comparative Transcriptome Analyses. Tropical Plant Biol. 10, 30–44 (2017). https://doi.org/10.1007/s12042-016-9182-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12042-016-9182-3