Abstract
A one-pot, solvent-free protocol for the synthesis of chloro-substituted benzoquinoline-carbazole derivatives via a modified Friedländer hetero-annulation reaction between 2, 3, 4, 9-tetrahydrocarbazol-1-one and 3-amino-2-naphthoic acid in the presence of \(\hbox {POCl}_{3}\) is described. In addition, the direct pseudo multicomponent transformation of 2, 3, 4, 9-tetrahydrocarbazol-1-one, malononitrile and 9-ethyl-3-carbazolecarboxaldehyde results in the formation of a multifunctionalized carbazole through a Knoevenagel–Michael addition-cyclization reaction has also been reported. All the newly synthesized molecules were deduced by spectral and analytical methods.
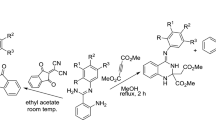
Graphical Abstract
A modified Friedländer hetero-annulation reaction between 2, 3, 4, 9-tetrahydro-1H-carbazol-1-one and 3-amino-2-naphthoic acid under solvent-free condition afforded chloro-substituted benzoquinoline-carbazole derivatives. In addition, the direct pseudo multicomponent transformation of 2, 3, 4, 9-tetrahydro-1H-carbazol-1-one, malononitrile and 9-ethyl-3-carbazolecarboxaldehyde results in the formation of dimerised carbazoles.







Similar content being viewed by others
References
Suresh J R, Syam Kumar U K, Ila H and Junjappa H 2001 Aromatic and heteroaromatic annelation studies on 3-[bis(methylthio)methylene]-1-methyloxindole: synthesis of carbazoles and an efficient route to pyrido[2,3-\(b\)]indoles Tetrahedron 57 781
Hu L X, Li Z R, Wang Y M, Wu Y B, Jiang J D and Boykin D W 2007 Novel pyridinyl and pyrimidinylcarbazole sulfonamides as antiproliferative agents Bioorg. Med. Chem. Lett. 17 1193
Dračínský M, Sejbal J, Rygerová B and Stiborová M 2007 An efficient modification of ellipticine synthesis and preparation of 13-hydroxyellipticine Tetrahedron Lett. 48 6893
Barbieri V and Ferlin M G 2006 Microwave-assisted one-pot synthesis of substituted tetrahydrocarbazole and 8,9,10,11-tetrahydro-7H-pyrido[a]carbazoles Tetrahedron Lett. 47 8289
Haider N, Jabara R, Khadami F and Wanko R 1998 Synthesis of Pyridazino [4, 5-\(b\)] carbazoles as potential antitumor agents Heterocycles 48 1609
Hedin V M, Tabka T, Poulain L, Godard T, Lachevrel M, Saturnino C, Lancelot J C, Le Talaer J Y and Gauduchon P 2000 Biological properties of 5,11-dimethyl-6H-pyrido [3, 2-b] carbazoles: a new class of potent antitumor drugs Anticancer Drug Des. 15 109
de Koning C B, Michael J P and Rosseau A L 2000 A versatile and convenient method for the synthesis of substituted benzo[a]carbazoles and pyrido[2,3-a]carbazoles J. Chem. Soc. Perkin Trans. 1 1705
Hirata K, Ito C, Furukawa H, Itogiawa M, Mark Cosentino L and Lee K H 1999 Substituted 7H-pyrido[4,3-c]carbazoles with potent anti-HIV activity Bioorg. Med. Chem. Lett. 9 119
Ohashi M, Shudo T, Nishijima K, Notso T, Kikuchi A, Yanagibashi K and Nishida H 2000 Pyridocarbazole derivatives having cGMP-PDE inhibitory activity, United States Patent 6018046 United States Mochida Pharmaceutical Co., Ltd, Tokyo, JP
Selvi G and Rajendran S P 2004 Synthesis of some new 2-[3-(2-chloro quinolinyl)]-3-aryl-4-thiazolidinones as potent antibacterial agents J. Asian Chem. 16 1017
Carr B A and Franklin M R 1998 Drug-metabolizing enzyme induction by 2,2\(^\prime \)-dipyridyl, 1,7-phenanthroline,7,8-benzoquinoline and oltipraz in mouse Xenobiotica 28 949
Mikhailitsyn F S, Kozyreva N P, Rabinovich S A, Maksakovskaya Y V, Kulikovskaya I M, Dadasheva N R, Lebedeva M N, Bekhli A F, Lychko N D and Uvarova N A 1992 Search for new antiparasitic agents, Synthesis, toxicity, and antimalarial effect of some nitrogen-containing heterocycles with 4-(4-alkylpiperazin-1-yl)phenylamino substituents Med. Parazitol. Parazit. Bolezni. 50
Nozulak J, Vigouret J M, Jaton A L, Hofmann A, Dravid A R, Weber H P, Kalkman H O and Walkinshaw M D 1992 Centrally acting al-adrenoceptor agonists based on hexahydronaphth[2,3-b]-1,4-oxazines and octahydrobenzo[g]quinolinesl J. Med. Chem. 35 480
Szmuszkovicz J, Darlington W H and Von Voigtlander P F 1988 Preparation and formulation of antipsychotic aminopolyhydrobenz(iso)quinolines and intermediates. WO 8804292 A1 1988 Chem. Abstr. 110 75335
Kantevari S, Yempala T, Surineni G, Sridhar B, Yogeeswari P and Sriram D 2011 Synthesis and antitubercular evaluation of novel dibenzo[b,d]furan and 9-methyl-9H-carbazole derived hexahydro-2H-pyrano[3,2-c]quinolines via Povarov reaction Eur. J. Med. Chem. 46 4827
(a) Prabha K and Rajendra Prasad K J 2016 Synthesis and Cytotoxic Distinction of Benzo[h]naphtho[1,2-b] [1,6] Naphthyridine and its Isomeric Benzo[b]naphtho[1,2-h][1,6] Naphthyridines Med. Chem. 62 062; (b) Indumathi T, Muthusankar A, Shanmughavel P and Rajendra Prasad K J 2013 Synthesis of hetero annulated carbazoles: exploration of in vitro cytotoxicity and molecular docking studies Med. Chem. Commun. 4 450; (c) Indumathi T, Jamal Ahamed V S, Surk-Sik Moon, Fronczek F R and Rajendra Prasad K J 2011 L-Proline anchored multicomponent synthesis of novel pyrido[2,3-a]carbazoles; investigation of in vitro antimicrobial, antioxidant, cytotoxicity and structure activity relationship studies Eur. J. Med. Chem. 46 5580; (d) Murali K, Sparkes H A and Rajendra Prasad K J 2017 Synthesis of hetero annulated isoxazolo-, pyrido- and pyrimido carbazoles: Screened for in vitro antitumor activity and structure activity relationships, a novel 2-amino-4-(3’-bromo-4’-methoxyphenyl)-8-chloro-11H-pyrimido[4,5-\(a\)]carbazole as an antitumor agent Eur. J. Med. Chem. 128 319; (e) Yamuna E, Yurcho A, Sovesky R J, Smith P M, Zeller M and Rajendra Prasad K J 2011 Elegant one-pot synthesis of quinolino[\(2{^\prime },3{^\prime }:7,6\)]-cyclohept[1,2-b]indole through Friedländer and Pfitzinger annulation reaction Synth. Commun. 41 3351
(a) Ryabukhin S V, Naumchik V S, Plaskon A S, Grygorenko O O and Tolmachev A A 2011 3-Haloquinolines by Friedländer reaction of r-haloketones J. Org. Chem. 76 5774; (b) Le Z-G, Liang M, Chen Z-S, Zhang S-H and Xie Z-B 2017 Ionic liquid as an efficient medium for the synthesis of quinoline derivatives via \(\alpha \)-chymotrypsin catalysed Friedländer condensation Molecules 22 762; (c) Tufail F, Saquib M, Singh S, Tiwari J, Singh M, Singh J and Singh J 2017 Bioorganopromoted green Friedländer synthesis: a versatile new malic acid promoted solvent free approach to multisubstituted quinoline New J. Chem. 41 1618
(a) Zhang X-L, Wang Q-Y, Sheng S-R, Wang Q and Liu X-L 2009 Efficient Friedländer Synthesis of quinoline derivatives from 2-aminoarylketones and carbonyl compounds mediated by recyclable PEG-supported sulfonic acid Synth. Commun. 39 3293; (b) Ramann G A and Cowen B J 2016 Recent advances in metal-free quinoline synthesis Molecules 21 986; (c) Reddy B P, Iniyavan P, Sarveswari S and Vijayakumar V 2014 Nickel oxide nanoparticles catalyzed synthesis of poly-substituted quinolines via Friedlander hetero-annulation reaction Chin. Chem. Lett. 25 1595
Luo W, Mu Q, Qiu W, Liu T, Yang F, Liu X and Tang J 2011 A novel Friedlander-type synthesis of 3-aryl quinolines from 3-oxo-2,3-diaryl-propionaldehydes Tetrahedron 67 7090
Genovese S, Epifano F, Marcotullio M C, Pelucchini C and Curini M 2011 An alternative quinoline synthesis by via Friedländer reaction catalyzed by Yb(OTf)3 Tetrahedron Lett. 52 3474
McNaughton B R and Miller B L 2003 A mild and efficient one-step synthesis of quinolines Org. Lett. 5 4257
Satheeshkumar R, Shanker R, Kaminsky W and Rajendra Prasad K J 2016 Novel synthetic and mechanistic approach of TFA catalyzed Friedlander synthesis of 2-acylquinolines from symmetrical and unsymmetrical 1,2-diketones with o-aminoarylketone ChemistrySelect. 1 6823
Prabakaran K and Rajendra Prasad K J 2010 Simple and convenient methods for the synthesis of indolo[3,2-c]acridines Synth. Commun. 40 3528
Ashok D, Ravi S, Vijaya Lakshmi B and Ganesh A 2015 One-pot synthesis of carbazole based 3-hydroxy-4H-chromen-4-ones by modified Algar–Flynn–Oyamada reaction and their antimicrobial activity J. Serb. Chem. Soc. 80 1361
(a) Song Y, Di C-A, Wei Z, Zhao T, Xu W, Liu Y, Zhang D and Zhu D 2008 Synthesis, characterization, and field-effect transistor properties of carbazolenevinylene oligomers: from linear to cyclic architectures Chem. Eur. J. 14 4731; (b) Simokaitiene J, Grigalevicius S, Grazulevicius J V, Rutkaite R, Kazlauskas K, Jursenas S, Jankauskas V and Sidaravicius J 2006 Synthesis, photophysical and photoelectrical properties of glass-forming phenothiazinyl-and carbazolyl-substituted ethylenes J. Optoelectron Adv. Mater. 8 876; (c) Jin Y, Zhang A, Huang Y and Zhang W 2010 Shape-persistent arylenevinylene macrocycles (AVMs) prepared via acyclic diene metathesis macrocyclization (ADMAC) Chem. Commun. 46 8258
Chen C H, Lin J T and Yeh M C P 2006 Stilbene like carbazole dimer-based electroluminescent materials Tetrahedron 62 8564
Jiao C-X, Shen Q, Huan S-Y, Shen G-L and Yu R-Q 2005 Conjugated carbazole dimer as fluorescence carrier for preparation of iodine-sensitive chemical sensor Anal. Chim. Acta 528 229
Murali K, Sparkes H A, Pandiyan B V and Rajendra Prasad K J 2017 Synthesis, photophysical properties and DFT analysis of highly substituted pyrido carbazole-based “push pull” chromophores New J. Chem. 41 8242
Maharani S and Ranjith Kumar R 2015 Domino four-component synthesis of novel cycloocta[b]pyridines Tetrahedraon Lett. 56 179
Acknowledgements
We would like to thank the University of Mysore, for NMR data. X-ray diffraction was funded by University of Bristol, United Kingdom. Financial support from “UGC-Emeritus fellowship” [Award NO.F.6-6/2015-17/EMERITUS-2015-17-OBC-7410/ (SAII)] for research, is gratefully acknowledged by Prof. K. J. Rajendra Prasad. Arya. K. R acknowledges the award of BSR-Senior Research Fellowship by University Grant Commission (UGC), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arya, K.R., Sparkes, H.A. & Prasad, K.J.P.R. An efficient one-pot synthesis of carbazole fused benzoquinolines and pyridocarbazoles. J Chem Sci 130, 41 (2018). https://doi.org/10.1007/s12039-018-1428-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1428-1