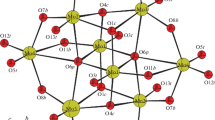
Abstract
The reaction of molybdic acid, phosphoric acid and copper(II) sulfate pentahydrate with m-toluidine in aqueous solution at room temperature furnished a new diphosphopentamolybdate (\(\hbox {C}_{7}\hbox {H}_{10}\hbox {N})_{4}[\hbox {H}_{2}\hbox {P}_{2}\hbox {Mo}_{5}\hbox {O}_{23}]\cdot \hbox {H}_{2}\hbox {O}\) (1). A single-crystal X-ray diffraction study showed that the compound crystallizes in the triclinic crystal system with space group \(P-1\) and unit cell constants, \(a~=~12.833\) (3) Å, \(b~=~13.9855\) (4) Å, \(c=14.9446\) (4) Å, \(\alpha =64.607\) (3)\({^{\circ }}\), \(\beta ~=~70.578\) (2)\({^{\circ }}\), \(\gamma \) = 65.713 (3)\({^{\circ }}\). The sample was also analyzed by energy dispersive spectroscopy (EDS), infrared spectroscopy (IR) and UV-visible spectroscopy. Using the refined atomic structure, Hirshfeld surface analysis and Semi-empirical calculations were performed to study the intermolecular interactions and calculate theoretical properties of 1.
Graphical Abstract
SYNOPSIS The title compound (\(\hbox {C}_{7}\hbox {H}_{10}\hbox {N})_{4}[\hbox {H}_{2}\hbox {P}_{2}\hbox {Mo}_{5}\hbox {O}_{23}]\cdot \hbox {H}_{2}\hbox {O}\) was synthesized and characterized by IR and UV spectroscopy, and its structure was solved by single crystal X-ray diffraction. The presence of the Mo, P, O, C and N atoms was confirmed by EDS analysis. The Hirshfeld surface analysis was performed to elucidate non-bonding interactions and theoretical properties were calculated.











Similar content being viewed by others
References
Tohomas J, Kannan K R and Ramanan A 2008 Nanostructured phosphomolybdates J. Chem. Sci. 120 529
Arumuganathan T, Siddikha A and Das S K 2017 ‘Ionic crystals’ consisting of trinuclear macrocations and polyoxometalate anions exhibiting single crystal to single crystal transformation: breathing of crystals J. Chem. Sci. 129 1121
Li S, Li Z, Zhang J, Su Z, Qi S, Guo S and Tan X 2017 Polyoxometalate-based 3D porous framework with inorganic molecular nanocage units J. Chem. Sci. 129 573
Hmida F, Ayed M, Ayed B and Haddad A 2015 Two new inorganic-organic hybrid materials based on inorganic cluster, [\(\text{ X }_{2}\text{ Mo }_{18}\text{ O }_{62}]^{6-}\) (X = P, As) J. Chem. Sci. 127 1645
Miras H N, Yan J, Long D L and Cronin L 2012 Engineering polyoxometalates with emergent properties Chem. Soc. Rev. 41 7403
Qu X, Xu L, Yang Y, Li F and Guo W 2011 Hydrothermal synthesis and crystal structure of (\(\text{ H }_{2}\text{ bpp })_{3}[\text{ Mo }_{5}\text{ P }_{2}\text{ O }_{23}]\text{ H }_{2}\text{ O }\): a twofold interpenetrating 3D supramolecular architecture constructed of Standberg-type polyoxometalate Struct. Chem. 22 965
Ganesan S V and Natarajan S 2005 Hydrothermal synthesis and structure of [(\(\text{ C }_{4}\text{ N }_{2}\text{ H }_{12})_{3}][\text{ P }_{2}\text{ Mo }_{5}\text{ O }_{23}]\cdot \text{ H }_{2}\text{ O }\) and [(\(\text{ C }_{3}\text{ N }_{2}\text{ H }_{12})_{3}][\text{ P }_{2}\text{ Mo }_{5}\text{ O }_{23}\)] \(\cdot \text{4H }_{2}\text{ O }\) J. Chem. Sci. 117 219
Liu H, Wang H, Niu D and Lu Z 2007 Microwave-Assisted Synthesis and Crystal Structure of Molybdophosphate Supramolecular Compound with 2-Aminopyridinium Cations Synth. Reac. Inorg. Met. Org. Nano-Met. Chem. 37 103
Asnani M, Kumar D, Duaisamy T and Ramanan A 2012 Crystallization of organically templated phosphomolybdate cluster-based solids from aqueous solution J. Chem. Sci. 124 1275
Palatinus L and Chapuis G 2007 SUPERFLIP - a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions J. Appl. Crystallogr. 40 786
Petricek V, Dusek M and Palatinus L 2014 Crystallographic Computing System JANA2006: General Features Z. Kristallogr. 229 345
Brandenburg K and Putz H 2005 DIAMOND Version 3. Crystal Impact GbR, Postfach 1251, D-53002 Bonn, Germany
Spartan 14 2014 Wavefunction Inc. Irvine CA 92,612, USA
Spackman M A and McKinnon J J 2002 Fingerprinting intermolecular interactions in molecular crystals CrystEngComm 4 378
Wolff S K, Grimwood D J, McKinnon J J, Turner M J, Jayatilaka D aand Spackman M A 2012 CRYSTAL-EXPLORER 3.0 University of Western Australia, Perth
Jayatilaka D, Grimwood D J and Lee A 2005 TONTO A System for Computational Chemistry (Nedlands: The University of Western Australia)
Hirshfeld F L 1977 Bonded-atom fragments for describing molecular charge densities Theor. Chim. Acta 44 129
Luo Y H, Mao Q X and Sun B W 2014 Two new complexes with 6-methylnicotinic acid ligand: Synthesis, crystal structure and Hirshfeld surfaces Inorg. Chim. Acta 412 60
Luo Y H, Sun B W 2013 Pharmaceutical co-crystals of pyrazinecarboxamide (PZA) with various carboxylic acids: crystallography, Hirshfeld surfaces, and dissolution study Cryst. Growth Des. 13 2098
Luo Y H, Xu Band Sun B W 2013 Investigation of supramolecular synthons of p-hydroxybenzoic acid (PHBA): Comparison of its hydrate, co-crystal and salt J. Cryst. Growth 374 88
Desiraju G R and Gavezzotti A 1989 Crystal structures of polynuclear aromatic hydrocarbons Classification, rationalization and prediction from molecular structure. Acta Crystallogr. Sec. B: Struct. Sci. 45 473
Prasad A A, Muthu K, Meenatchi V, Rajasekar M, Agilandeshwari R, Meena K, Manonmoni J V and Meenakshisundaram S P 2015 Optical, vibrational, NBO, first-order molecular hyperpolarizability and Hirshfeld surface analysis of a nonlinear optical chalcone Spectrochim. Acta Part A: Mol. Biomol. Spect. 140 311
Ammari Y, Dhahri E, Rzaigui M, Hlil E K and Abid S 2016 Synthesis, structure and physical properties of an hybrid compound based on Strandberg type polyoxoanions and copper cations J. Clust. Sci. 27 1213
He X, Zhang P, Song T Y, Mu Z C, Yu J H, Wang Y and Xu J N 2004 Hydrothermal synthesis and structure of a molybdenum(VI) phosphate cluster and a three dimensional cobalt molybdenum(V) phosphate Polyhedron 23 2153
Wei C X, Chen J X, Huang Y B, Lan T Y, Li Z S, Zhang W J and Zhang Z C 2006 Syntheses, structures and properties of two molybdenum phosphates [(\(\text{ H }_{20}\text{ P }_{8}\text{ Mo }^{V}_{12}\text{ CdO }_{62}\)) (\(\text{ C }_{4}\text{ H }_{14}\text{ N }_{3})_{2}]\text{2C }_{4}\text{ H }_{13}\text{ N }_{3}\text{8H }_{2}\text{ O }\) and [(\(\text{ H }_{2}\text{ P }_{2}\text{ Mo }^{VI}_{5} \text{ O }_{23})\) (\(\text{ C }_{4}\text{ H }_{14}\text{ N }_{3})\) (\(\text{ C }_{4}\text{ H }_{15}\text{ N }_{3})\) (\(\text{ H }_{3}\text{ O })]\text{3H }_{2}\text{ O }\) J. Mol. Struct. 798 117
Ma Y, Lu Y, Wang E, Xu X, Guo Y, Bai X and Xu L 2006 Hydrothermal synthesis and crystal structure of a novel three-dimensional supramolecular network containing cyclic water hexamers: [\(\text{ Co(en) }_{3}]4[\text{ P }_{2}\text{ Mo }_{5}\text{ O }_{23}]_{2}\text{9H }_{2}\text{ O }\) (en = ethylenediamine) J. Mol. Struct. 784 18
Wang Y, Zhang L C, Zhu Z M, Li N, Deng A F and Zheng S Y 2011 Assembly of four copper(II)–2,2’-biimidazole complex-supported Strandberg-type phosphomolybdates Transit. Met. Chem. 36 261
Nagazi I and Haddad A 2012 Synthesis, characterization and crystal structure of a novel Strandberg-type polyoxoselenomolybdate \(\text{ Rb }_{4}[\text{ Se }_{2}\text{ Mo }_{5}\text{ O }_{21}]\text{2H }_{2}\text{ O }\) Mat. Res. Bull. 47 356
Zhao J W, Li Y Y, Wang Y H, Shi D Y, Luo J and Chen L J 2013 A novel 2D phosphomolybdate hybrid [\(\text{ Cu(En)(EnH) }]_{2}[\text{ P }_{2}\text{ Mo }_{5}\text{ O }_{23}]\text{3H }_{2}\text{ O }\) constructed from strandberg-type polyoxometalate units and copper-organic cation bridges Rus. J. Coord. Chem. 39 519
Sharma S, Brahmachari G, Banerjee B, Nurjamal K, Kumar A K, Srivastava A, Misra N, Pandey S K, Rajnikant and Gupta V K 2016 Synthesis, spectroscopic characterization and crystallographic behavior of a biologically relevant novel indole-fused heterocyclic compound — Experimental and theoretical (DFT) studies J. Mol. Struct. 1118 344
Soria-Martínez R, Mendoza-Merono R and García-Granda S 2016 Synthesis, crystal structure, spectroscopic characterization and theoretical study of (2E)-N-phenyl-2-(pyridin-3-ylmethylidene)hydrazinecarboxamide J. Mol. Struct. 1105 322
Acknowledgements
This work was supported by the Ministry of Higher Education and Scientific Research of Tunisia. The crystallographic part was supported by the project 15-12653S of the Czech Science Foundation using instruments of the ASTRA lab established within the Operation program Prague Competitiveness - Project CZ.2.16/3.1.00/24510.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Harchani, A., Kučeráková, M., Dušek, M. et al. Synthesis, characterization, Hirshfeld surface and theoretical properties of \((\hbox {C}_{7}\hbox {H}_{10}\hbox {N})_{4} [\hbox {H}_{2}\hbox {P}_{2}\hbox {Mo}_{5}\hbox {O}_{23}]\cdot \hbox {H}_{2}\hbox {O}\) . J Chem Sci 129, 1539–1547 (2017). https://doi.org/10.1007/s12039-017-1361-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-017-1361-8