Abstract
The electrochemical behaviour of Cu(II)/Cu(I) redox couple in 1-hexyl-3-methylimidazolium chloride (C6mimCl) ionic liquid was studied using glassy carbon electrode at 375 K by cyclic voltammetry, chronopotentiometry and electrochemical impedance spectroscopy. In this electrochemical study, we have made an attempt to avoid the problem of water contamination in hygroscopic C6mimCl ionic liquid by setting the temperature at 375 K without glove box. This high temperature cyclic voltammetric study revealed two-step one electron reductions of Cu(II) to Cu(I) followed by Cu(I) to Cu metal. The reduction of Cu(II) to Cu(I) was found to be quasi-reversible at 375 K. The diffusion coefficients of Cu(II) and Cu(I), and the charge transfer rate constant of Cu(II) in C6mimCl were estimated by Randles-Ševčik equation and Nicholson’s method, respectively, and found to be consistent with the quasi-reversible process. Further, constant potential electrodeposition of metallic copper was carried out on a stainless steel electrode at 375 K and the deposit was characterised by X-ray diffraction and electron microscopy.

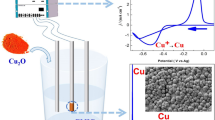
Quasi-reversible behavior of Cu(II)/Cu(I) redox couple in C6mimCl is studied at 375 K. Diffusion and mass transport coefficients as well as charge transfer rate constant of copper species are estimated. Copper on stainless-steel electrodes by constant potential electrolysis shows island growth of Cu particles under the influence of C6mimCl.







Similar content being viewed by others
References
Galinski M, Lewandowski A and Stepniak I 2006 Electrochim. Acta 51 5567
Abbott A P and McKenzie K 2006 J. Phys. Chem. Chem. Phys. 8 4265
Endres F, Mac Farlane D and Abbott A P (eds.) 2008 Electrodeposition from Ionic Liquids (Germany: Wiley ACH)
Venkatesan K A, Rao C J, Nagarajan K and Rao P R V 2012 Int. J. Electrochem. Article ID 841456, 12 pages, doi: 10.1155/2012/841456
KatayamaY 2011 In Electrochemical aspects of ionic liquids 2nd ed. H Ohno (Ed.) (New Jersey: John Wiley)
Rao C J, Venkatesan K A, Nagarajan K, Srinivasan T G and Rao P R V 2009 Electrochim. Acta 54 4718
Simka W, Puszczyk D and Nawrat G 2009 Electrochim. Acta 54 5307
Endres F 2002 ChemPhysChem 3 144
Welton T 1999 Chem. Rev. 99 2071
Earle M J and Seddon K R 2000 Pure Appl. Chem. 72 1391
Freemantle M 2010 In An introduction to Ionic Liquids (Cambridge: RSC Publishing)
Plechkova N V and Seddon K R 2008 Chem. Soc. Rev. 37 123
Earle M J, Esperanc J M S S, Gilea M A, Lopes J N C, Rebelo L P N, Magee J W, Seddon K R and Widegren J A 2006 Nature 439 831
Ngo H L, Compte K L, Hargens L and McEwen A B 2000 Thermochim. Acta 357 97
Pandey S 2006 Anal. Chim. Acta 556 38
Rao P R V, Venkatesan K A and Srinivasan T G 2008 Prog. Nucl. Energy 50 449
Sakaebe H, Matsumoto H and Tatsumi K 2007 Electrochim. Acta 53 1048
Hu C, Guo Y, Wang J, Yang L, Yang Z, Bai Z, Zhang J, Wang K and Jiang K 2012 ACS Appl. Mater. Interfaces 4 4461
Yang C, Kumar A S, Kuo M, Chien S and Zena J 2005 AnalyticaChimicaActa 554 66
Reyter D, Belanger D and Roue L 2009 J. Phys. Chem. C 113 290
Tang W, Zhang L and Henkelman G 2011 J. Phys. Chem. Lett. 2 1328
Hussey C L, King L A and Carpio R A 1979 J. Electrochem. Soc.: Solid-state Science and Technology 1029
Nanjundiah C and Osteryoung R A 1983 J. Electrochem. Soc.: Electrochemical Science and Technology 130 1312
Chen P Y and Sun I W 1999 Electrochim. Acta 45 441
Assaker I B and Dhahbi M 2011 J. Mol. Liq. 161 13
Chen P Y and ChangY 2012 Electrochim. Acta 75 339
El Abedin S Z, Saad A Y, Farag H K, Borisenko N, Liu Q X and Endres F 2007 Electrochim. Acta 52 2746
Moeller T 1944 J. Phys. Chem. 48 111
Huddleston J G, Visser A E, Reichert W M, Willauer H D, Broker G A and Rogers R D 2001 Green Chem. 3 156
Bard A J and Faulkner L R 2001 In Electrochemical Methods: Fundamentals and Applications 2nd ed. (New York: John Wiley)
Scholz F (Ed.) 2010 In Electroanalytical Methods 2nd ed. (Berlin: Springer-Verlag)
Nagaishi R, Arisaka M, Kimura T and Kitatsuji Y 2007 J. Alloys Compd. 431 221
Kuznetsov S A, Hayashi H, Minato K and Escard M G 2005 J. Electrochem. Soc. 152 C203
Kuznetsov S A and Escard M G 2006 J. Electroanal. Chem. 595 11
Nicholson R S 1965 Anal. Chem. 37 1351
Ramamurthy A C and Rangarajan S K 1981 Electrochim. Acta 26 111
Compton R G and Banks C E 2011 In Understanding Voltammetry 2nd ed. (London: Imperial College Press)
Barsoukov E and Macdonald J R (Eds.) 2005 In Impedance Spectroscopy 2nd ed. (New Jersey: John Wiley)
Acknowledgement
We thank MNRE and DST, New Delhi, for providing financial support to establish the experimental facilities for this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
The electronic supporting information can be seen at www.ias.ac.in/chemsci.
A. Sri Dithya is Science Academies’ Summer Research Fellow in 2012
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
SHAKEELA, K., DITHYA, A.S., RAO, C.J. et al. Electrochemical behaviour of Cu(II)/Cu(I) redox couple in 1-hexyl-3-methylimidazolium chloride ionic liquid. J Chem Sci 127, 133–140 (2015). https://doi.org/10.1007/s12039-014-0758-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0758-x