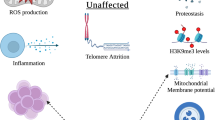
Abstract
It has been proposed that age reprogramming enables old cells to be rejuvenated without passage through an embryonic stage (Singh and Zacouto in J Biosci 35:315–319, 2010). As such, age reprogramming stands apart from the induced pluripotent stem (iPS) and nuclear transfer-embryonic stem (NT-ES) cell therapies where histo-compatible cells are produced only after passage through an embryonic stage. It avoids many of the disadvantages associated with iPS and NT-ES cell therapies. Experimental evidence in support of age reprogramming is burgeoning. Here, we discuss possible new approaches to enhance age reprogramming, which will have considerable benefits for regenerative therapies.



Similar content being viewed by others
References
Abad M, Mosteiro L, Pantoja C, Canamero M, Rayon T, Ors I, Grana O, Megias D, Domínguez O, Martínez D, Manzanares M 2013 Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 502 340–345
Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J and Ding S 2011 Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat. Cell Biol. 13 215–222
Guo L, Karoubi G, Duchesneau P, Shutova MV, Sung HK, Tonge P, Bear C, Rogers I, Nagy A, Waddell TK 2017 Generation of induced progenitor-like cells from mature epithelial cells using interrupted reprogramming. Stem Cell Rep. 9 1780–1795
Gurdon JB and Melton DA 2008 Nuclear reprogramming in cells. Science 322 1811–1815
Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, Deconde R 2013 Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 49 359–367
Horvath S 2013 DNA methylation age of human tissues and cell types. Genome Biol. 14 R115
Huh CJ, Zhang B, Victor MB, Dahiya S, Batista LF, Horvath S and Yoo AS 2016 Maintenance of age in human neurons generated by microRNA-based neuronal conversion of fibroblasts. Elife 5 e18648
Kim Y, Zheng X, Ansari Z, Bunnell MC, Herdy JR, Traxler L, Lee H, Paquola ACM, Blithikioti C, Ku M, Schlachetzki JC 2018 Mitochondrial aging defects emerge in directly reprogrammed human neurons due to their metabolic profile. Cell Rep. 23 2550–2558
Ladewig J, Mertens J, Kesavan J, Doerr J, Poppe D, Glaue F, Herms S, Wernet P, Kögler G, Müller FJ, Koch P 2012 Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat. Methods 9 575–578
Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Ait-Hamou N, Leschik J, Pellestor F Ramirez JM, De Vos J, Lehmann S 2011 Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 25 2248–2253
Lopez-Otin C, Blasco MA, Partridge L, Serrano M and Kroemer G 2013 The hallmarks of aging. Cell 153 1194–1217
Manukyan M and Singh PB 2012 Epigenetic rejuvenation. Genes Cells 17 337–343
Manukyan M and Singh PB 2014 Epigenome rejuvenation: HP1beta mobility as a measure of pluripotent and senescent chromatin ground states. Sci. Rep. 4 4789
Mizutani E, Ono T, Li C, Maki-Suetsugu R and Wakayama T 2008 Propagation of senescent mice using nuclear transfer embryonic stem cell lines. Genesis 46 478–483
Nagy A and Nagy K 2010 The mysteries of induced pluripotency: where will they lead? Nat. Methods 7 22–24
Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M, Beyret E, Araoka T 2016 In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 167 1719–1733
Ohnishi K, Semi K, Yamamoto T, Shimizu M, Tanaka A, Mitsunaga K, Okita K, Osafune K, Arioka Y, Maeda T, Soejima H 2014 Premature termination of reprogramming in vivo leads to cancer development through altered epigenetic regulation. Cell 156 663–677
Ohnuki M, Tanabe K, Sutou K, Teramoto I, Sawamura Y, Narita M, Nakamura M, Tokunaga Y, et al. 2014 Dynamic regulation of human endogenous retroviruses mediates factor-induced reprogramming and differentiation potential. Proc. Natl. Acad. Sci. USA 111 12426–12431
Olova N, Simpson DJ, Marioni RE and Chandra T 2019 Partial reprogramming induces a steady decline in epigenetic age before loss of somatic identity. Aging Cell 18 e12877
Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Südhof TC, Wernig M 2011 Induction of human neuronal cells by defined transcription factors. Nature 476 220–223
Sheng C, Jungverdorben J, Wiethoff H, Lin Q, Flitsch LJ, Eckert D, Hebisch M, Fischer J, Kesavan J, Weykopf B, Schneider L 2018 A stably self-renewing adult blood-derived induced neural stem cell exhibiting patternability and epigenetic rejuvenation. Nat Commun. 9 4047
Singh PB and Newman A G 2018 Age reprogramming and epigenetic rejuvenation. Epigenet. Chromatin 11 73
Singh PB and Zacouto F 2010 Nuclear reprogramming and epigenetic rejuvenation. J. Biosci. 35 315–319
Tang Y, Liu ML, Zang T and Zhang CL 2017 Direct reprogramming rather than iPSC-based reprogramming maintains aging hallmarks in human motor neurons. Front. Mol. Neurosci. 10 359
Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC and Wernig M 2010 Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463 1035–1041
Yamanaka S and Blau HM 2010 Nuclear reprogramming to a pluripotent state by three approaches. Nature 465 704–712
Acknowledgements
This work was supported by a grant from the Ministry of Education and Science of Russian Federation #14.Y26.31.0024 and Nazarbayev University (Grant No. 090118FD5311).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, P.B., Laktionov, P.P. & Newman, A.G. Deconstructing age reprogramming. J Biosci 44, 106 (2019). https://doi.org/10.1007/s12038-019-9923-1
Published:
DOI: https://doi.org/10.1007/s12038-019-9923-1