Abstract
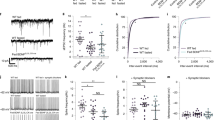
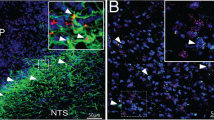
The hypothalamus is involved in the regulation of food intake and energy homeostasis. The arcuate nucleus (ARC) and median eminence (ME) are the primary hypothalamic sites that sense leptin and nutrients in the blood, thereby mediating food intake. Recently, studies demonstrating a role for non-neuronal cell types, including astrocytes and tanycytes, in these regulatory processes have begun to emerge. However, the molecular mechanisms involved in these activities remain largely unknown. In this study, we examined in detail the localization of fatty acid-binding protein 7 (FABP7) in the hypothalamic ARC and sought to determine its role in the hypothalamus. We performed a phenotypic analysis of diet-induced FABP7 knockout (KO) obese mice and of FABP7 KO mice treated with a single leptin injection. Immunohistochemistry revealed that FABP7+ cells are NG2+ or GFAP+ in the ARC and ME. In mice fed a high-fat diet, weight gain and food intake were lower in FABP7 KO mice than in wild-type (WT) mice. FABP7 KO mice also had lower food intake and weight gain after a single injection of leptin, and we consistently confirmed that the number of pSTAT3+ cells in the ARC indicated that the leptin-induced activation of neurons was significantly more frequent in FABP7 KO mice than in WT mice. In FABP7 KO mice-derived primary astrocyte cultures, the level of ERK phosphorylation was lower after leptin treatment. Collectively, these results indicate that in hypothalamic astrocytes, FABP7 might be involved in sensing neuronal leptin via glia-mediated mechanisms and plays a pivotal role in controlling systemic energy homeostasis.






Similar content being viewed by others
References
Myers MG Jr, Olson DP (2012) Central nervous system control of metabolism. Nature 491(7424):357–363. https://doi.org/10.1038/nature11705
Rodriguez EM, Blazquez JL, Guerra M (2010) The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: The former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides 31(4):757–776. https://doi.org/10.1016/j.peptides.2010.01.003
Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404(6778):661–671. https://doi.org/10.1038/35007534
Cone RD (2005) Anatomy and regulation of the central melanocortin system. Nat Neurosci 8(5):571–578. https://doi.org/10.1038/nn1455
Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ (2001) Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411(6836):480–484. https://doi.org/10.1038/35078085
Lin HV, Plum L, Ono H, Gutierrez-Juarez R, Shanabrough M, Borok E, Horvath TL, Rossetti L et al (2010) Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes 59(2):337–346. https://doi.org/10.2337/db09-1303
Ghasemi R, Haeri A, Dargahi L, Mohamed Z, Ahmadiani A (2013) Insulin in the brain: Sources, localization and functions. Mol Neurobiol 47(1):145–171. https://doi.org/10.1007/s12035-012-8339-9
Marty N, Dallaporta M, Thorens B (2007) Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda) 22:241–251. https://doi.org/10.1152/physiol.00010.2007
Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE (2004) Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53(3):549–559
Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M et al (2003) The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37(4):649–661
van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D (2004) Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci 7(5):493–494. https://doi.org/10.1038/nn1226
Argente-Arizon P, Freire-Regatillo A, Argente J, Chowen JA (2015) Role of non-neuronal cells in body weight and appetite control. Front Endocrinol 6:42. https://doi.org/10.3389/fendo.2015.00042
Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ (2008) Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology 149(6):2798–2806. https://doi.org/10.1210/en.2007-1673
Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, Fossier PB, Pan W (2009) Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain : J Neurol 132(Pt 4):889–902. https://doi.org/10.1093/brain/awp029
Kim JG, Suyama S, Koch M, Jin S, Argente-Arizon P, Argente J, Liu ZW, Zimmer MR et al (2014) Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci 17(7):908–910. https://doi.org/10.1038/nn.3725
Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J et al (2010) Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A 107(33):14875–14880. https://doi.org/10.1073/pnas.1004282107
Pan W, Hsuchou H, Xu C, Wu X, Bouret SG, Kastin AJ (2011) Astrocytes modulate distribution and neuronal signaling of leptin in the hypothalamus of obese a vy mice. J Mol Neurosci : MN 43(3):478–484. https://doi.org/10.1007/s12031-010-9470-6
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA et al (2012) Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122(1):153–162. https://doi.org/10.1172/JCI59660
Martin-Jimenez CA, Gaitan-Vaca DM, Echeverria V, Gonzalez J, Barreto GE (2016) Relationship between obesity, Alzheimer's disease, and Parkinson's disease: An Astrocentric view. Mol Neurobiol 54:7096–7115. https://doi.org/10.1007/s12035-016-0193-8
Ockner RK, Manning JA, Poppenhausen RB, Ho WK (1972) A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science 177(4043):56–58
Alpers DH, Strauss AW, Ockner RK, Bass NM, Gordon JI (1984) Cloning of a cDNA encoding rat intestinal fatty acid binding protein. Proc Natl Acad Sci U S A 81(2):313–317
Tweedie S, Edwards Y (1989) cDNA sequence for mouse heart fatty acid binding protein, H-FABP. Nucleic Acids Res 17(11):4374
Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, Muller T (1994) The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development 120(9):2637–2649
Haunerland NH, Spener F (2004) Fatty acid-binding proteins--insights from genetic manipulations. Prog Lipid Res 43(4):328–349. https://doi.org/10.1016/j.plipres.2004.05.001
Chmurzynska A (2006) The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J Appl Genet 47(1):39–48. https://doi.org/10.1007/BF03194597
Furuhashi M, Hotamisligil GS (2008) Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7(6):489–503. https://doi.org/10.1038/nrd2589
Wolfrum C, Borrmann CM, Borchers T, Spener F (2001) Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors alpha - and gamma-mediated gene expression via liver fatty acid binding protein: A signaling path to the nucleus. Proc Natl Acad Sci U S A 98(5):2323–2328. https://doi.org/10.1073/pnas.051619898
Glatz JF, Borchers T, Spener F, van der Vusse GJ (1995) Fatty acids in cell signalling: Modulation by lipid binding proteins. Prostaglandins Leukot Essent Fat Acids 52(2–3):121–127
Jefferson JR, Powell DM, Rymaszewski Z, Kukowska-Latallo J, Lowe JB, Schroeder F (1990) Altered membrane structure in transfected mouse L-cell fibroblasts expressing rat liver fatty acid-binding protein. J Biol Chem 265(19):11062–11068
Sharifi K, Morihiro Y, Maekawa M, Yasumoto Y, Hoshi H, Adachi Y, Sawada T, Tokuda N et al (2011) FABP7 expression in normal and stab-injured brain cortex and its role in astrocyte proliferation. Histochem Cell Biol 136(5):501–513. https://doi.org/10.1007/s00418-011-0865-4
Kagawa Y, Yasumoto Y, Sharifi K, Ebrahimi M, Islam A, Miyazaki H, Yamamoto Y, Sawada T et al (2015) Fatty acid-binding protein 7 regulates function of caveolae in astrocytes through expression of caveolin-1. Glia 63(5):780–794. https://doi.org/10.1002/glia.22784
Owada Y, Abdelwahab SA, Kitanaka N, Sakagami H, Takano H, Sugitani Y, Sugawara M, Kawashima H et al (2006) Altered emotional behavioral responses in mice lacking brain-type fatty acid-binding protein gene. Eur J Neurosci 24(1):175–187. https://doi.org/10.1111/j.1460-9568.2006.04855.x
Maniscalco JW, Rinaman L (2014) Systemic leptin dose-dependently increases STAT3 phosphorylation within hypothalamic and hindbrain nuclei. Am J Physiol Regul Integr Comp Physiol 306(8):R576–R585. https://doi.org/10.1152/ajpregu.00017.2014
Ellacott KL, Halatchev IG, Cone RD (2006) Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology 147(7):3190–3195. https://doi.org/10.1210/en.2005-0877
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS et al (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445(7124):168–176. https://doi.org/10.1038/nature05453
Cortes-Campos C, Elizondo R, Carril C, Martinez F, Boric K, Nualart F, Garcia-Robles MA (2013) MCT2 expression and lactate influx in anorexigenic and orexigenic neurons of the arcuate nucleus. PLoS One 8(4):e62532. https://doi.org/10.1371/journal.pone.0062532
Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn-Meynell AA, Balland E, Lacombe A et al (2013) Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab 17(4):607–617. https://doi.org/10.1016/j.cmet.2013.03.004
Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falluel-Morel A et al (2014) Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab 19(2):293–301. https://doi.org/10.1016/j.cmet.2013.12.015
Broadwell RD, Brightman MW (1976) Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. J Comp Neurol 166(3):257–283. https://doi.org/10.1002/cne.901660302
Faouzi M, Leshan R, Bjornholm M, Hennessey T, Jones J, Munzberg H (2007) Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology 148(11):5414–5423. https://doi.org/10.1210/en.2007-0655
Norsted E, Gomuc B, Meister B (2008) Protein components of the blood-brain barrier (BBB) in the mediobasal hypothalamus. J Chem Neuroanat 36(2):107–121. https://doi.org/10.1016/j.jchemneu.2008.06.002
Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L (2002) Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51(2):271–275
Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L (2005) Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11(3):320–327. https://doi.org/10.1038/nm1201
Pocai A, Lam TK, Obici S, Gutierrez-Juarez R, Muse ED, Arduini A, Rossetti L (2006) Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest 116(4):1081–1091. https://doi.org/10.1172/JCI26640
Le Foll C, Dunn-Meynell A, Musatov S, Magnan C, Levin BE (2013) FAT/CD36: A major regulator of neuronal fatty acid sensing and energy homeostasis in rats and mice. Diabetes 62(8):2709–2716. https://doi.org/10.2337/db12-1689
Taib B, Bouyakdan K, Hryhorczuk C, Rodaros D, Fulton S, Alquier T (2013) Glucose regulates hypothalamic long-chain fatty acid metabolism via AMP-activated kinase (AMPK) in neurons and astrocytes. J Biol Chem 288(52):37216–37229. https://doi.org/10.1074/jbc.M113.506238
Young JK (2002) Anatomical relationship between specialized astrocytes and leptin-sensitive neurones. J Anat 201(1):85–90. https://doi.org/10.1046/j.1469-7580.2002.00068.x
Ebrahimi M, Yamamoto Y, Sharifi K, Kida H, Kagawa Y, Yasumoto Y, Islam A, Miyazaki H et al (2016) Astrocyte-expressed FABP7 regulates dendritic morphology and excitatory synaptic function of cortical neurons. Glia 64(1):48–62. https://doi.org/10.1002/glia.22902
Garcia-Caceres C, Fuente-Martin E, Burgos-Ramos E, Granado M, Frago LM, Barrios V, Horvath T, Argente J et al (2011) Differential acute and chronic effects of leptin on hypothalamic astrocyte morphology and synaptic protein levels. Endocrinology 152(5):1809–1818. https://doi.org/10.1210/en.2010-1252
Fuente-Martin E, Garcia-Caceres C, Granado M, de Ceballos ML, Sanchez-Garrido MA, Sarman B, Liu ZW, Dietrich MO et al (2012) Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest 122(11):3900–3913. https://doi.org/10.1172/JCI64102
Gao S, Kinzig KP, Aja S, Scott KA, Keung W, Kelly S, Strynadka K, Chohnan S et al (2007) Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc Natl Acad Sci U S A 104(44):17358–17363. https://doi.org/10.1073/pnas.0708385104
Chen N, Sugihara H, Kim J, Fu Z, Barak B, Sur M, Feng G, Han W (2016) Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. elife 5. https://doi.org/10.7554/eLife.18716
Sweeney P, Qi Y, Xu Z, Yang Y (2016) Activation of hypothalamic astrocytes suppresses feeding without altering emotional states. Glia 64(12):2263–2273. https://doi.org/10.1002/glia.23073
Dawson MR, Polito A, Levine JM, Reynolds R (2003) NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24(2):476–488
Peters A (2004) A fourth type of neuroglial cell in the adult central nervous system. J Neurocytol 33(3):345–357. https://doi.org/10.1023/B:NEUR.0000044195.64009.27
Robins SC, Trudel E, Rotondi O, Liu X, Djogo T, Kryzskaya D, Bourque CW, Kokoeva MV (2013) Evidence for NG2-glia derived, adult-born functional neurons in the hypothalamus. PLoS One 8(10):e78236. https://doi.org/10.1371/journal.pone.0078236
Djogo T, Robins SC, Schneider S, Kryzskaya D, Liu X, Mingay A, Gillon CJ, Kim JH et al (2016) Adult NG2-glia are required for median eminence-mediated leptin sensing and body weight control. Cell Metab 23(5):797–810. https://doi.org/10.1016/j.cmet.2016.04.013
Sharifi K, Ebrahimi M, Kagawa Y, Islam A, Tuerxun T, Yasumoto Y, Hara T, Yamamoto Y et al (2013) Differential expression and regulatory roles of FABP5 and FABP7 in oligodendrocyte lineage cells. Cell Tissue Res 354(3):683–695. https://doi.org/10.1007/s00441-013-1730-7
Acknowledgments
We thank Professor W. Stallcup for gifting the anti-NG2 and anti-PDGFRα antibodies. This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 16K12735, 16H06616 and Tohoku University Center for the Gender Equality Promotion Start-up Grant.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All experimental procedures involving mice were approved by the Institute of Laboratory Animals of Tohoku University Graduate School of Medicine and carried out according to the Guidelines for Animal Experimentation of the Tohoku University Graduate School of Medicine and according to the laws and notification requirements of the Japanese government.
Electronic supplementary material
Supplemental Fig. 1
WAT and T-CHO were significantly lower in FABP7 KO mice than in WT mice fed a HFD for 12 weeks. (a) Weight of EWAT (g), (b-f) Serum blood glucose (mg/dL), TG (mg/dL), FFA (mg/dL), T-CHO (mg/dL), and ALT (mg/dL) levels in WT and FABP7 KO mice fed a control diet or HFD. The data are presented as the mean ± SEM (n = 5) and are representative of three independent experiments. *p < 0.05, analyzed using Student’s t tests. (GIF 54 kb)
Supplemental Fig. 2
HFD did not alter FABP7 levels in the ARC and ME. (a) qPCR results showing the expression of FABP7 in the ARC and ME. The data are presented as the mean ± SEM (n = 4) and were analyzed using Student’s t tests. STD: standard diet, HFD: high fat diet (b) The total number of FABP7+ cells in the ARC. The data are presented as the mean ± SEM in 4 sections (n = 4) and were analyzed using Student’s t tests. (c) Representative images of FABP7 labeling in the ARC and ME. Scale bars = 50 μm. (GIF 89 kb)
Rights and permissions
About this article
Cite this article
Yasumoto, Y., Miyazaki, H., Ogata, M. et al. Glial Fatty Acid-Binding Protein 7 (FABP7) Regulates Neuronal Leptin Sensitivity in the Hypothalamic Arcuate Nucleus. Mol Neurobiol 55, 9016–9028 (2018). https://doi.org/10.1007/s12035-018-1033-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1033-9