Abstract
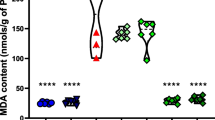
The presence of oxidative stress in immature brain has been demonstrated during the acute phase of status epilepticus (SE). The knowledge regarding the long periods of survival after SE is not unequivocal, lacking direct evidence. To examine the presence and time profile of oxidative stress, its functional effect on mitochondria and the influence of an antioxidant treatment in immature rats during epileptogenesis, status epilepticus (SE) was induced in immature 12-day-old rats by Li-pilocarpine and at selected periods of the epileptogenesis; rat pups were subjected to examinations. Hydroethidine method was employed for detection of superoxide anion (O2.−), 3-nitrotyrosine (3-NT), and 4-hydroxynonenal (4-HNE) for oxidative damage of mitochondrial proteins and complex I activity for mitochondrial function. Natural polyphenolic antioxidant resveratrol was given in two schemes: “acute treatment,” i.p. administration 30 min before, 30 and 60 min after induction of SE and “full treatment” when applications continued once daily for seven consecutive days (25 mg/kg each dose). The obtained results clearly document that the period of epileptogenesis studied (up to 4 weeks) in immature brain is associated with the significant enhanced production of O2.−, the increased levels of 3-NT and 4-HNE and the persisting deficiency of complex I activity. Application of resveratrol either completely prevented or significantly reduced markers both of oxidative stress and mitochondrial dysfunction. The findings suggest that targeting oxidative stress in combination with current antiepileptic therapies may provide a benefit in the treatment of epilepsy.




Similar content being viewed by others
References
Lynch M, Sayin U, Bownds J, Janumpalli S, Sutula T (2000) Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. Eur J Neurosci 12:2252–2264. https://doi.org/10.1046/j.1460-9568.2000.00117.x
Kubová H, Mareš P, Suchomelová L, Brožek G, Druga R, Pitkänen A (2004) Status epilepticus in immature rats leads to behavioural and cognitive impairment and epileptogenesis. Eur J Neurosci 19(12):3255–3265. https://doi.org/10.1111/j.0953-816X.2004.03410.x
Pitkänen A, Lukasiuk K (2011) Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol 10(2):173–186. https://doi.org/10.1016/S1474-4422(10)70310-0
Patel M (2004) Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic Biol Med 37:951–962. https://doi.org/10.1016/j.freeradbiomed.2004.08.021.
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7134):787–795. https://doi.org/10.1038/nature05592
Waldbaum S, Patel M (2010) Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Res 88(1):23–45. https://doi.org/10.1016/j.eplepsyres.2009.09.020
Shin E-J, Jeong JH, Chung YH, Kim W-K, Ko K-H, Bach J-H, Hong J-S, Yoneda Y et al (2011) Role of oxidative stress in epileptic seizures. Neurochem Int 59(2):122–137. https://doi.org/10.1016/j.neuint.2011.03.025
Folbergrová J, Kunz WS (2012) Mitochondrial dysfunction in epilepsy. Mitochondrion 12(1):35–40. https://doi.org/10.1016/j.mito.2011.04.004
Liang LP, Waldbaum S, Rowley S, Huang TT, Day BJ, Patel M (2012) Mitochondrial oxidative stress and epilepsy in SOD2 deficient mice: Attenuation by a lipophilic metalloporphyrin. Neurobiol Dis 45(3):1068–1076. https://doi.org/10.1016/j.nbd.2011.12.025
Rowley S, Patel M (2013) Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med 62:121–131. https://doi.org/10.1016/j.freeradbiomed.2013.02.002
Folbergrová J (2013) Oxidative stress in immature brain following experimentally-induced seizures. Physiol Res 62(Suppl.1):S39–S48
Folbergrová J, Druga R, Otáhal J, Haugvicová R, Mareš P, Kubová H (2006) Effect of free radical spin trap N-tert-butyl-α-phenylnitrone (PBN) on seizures induced in immature rats by homocysteic acid. Exp Neurol 201(1):105–119. https://doi.org/10.1016/j.expneurol.2006.03.031
Folbergrová J, Ješina P, Drahota Z, Lisý V, Haugvicová J, Vojtíšková A, Houštěk J (2007) Mitochondrial complex I inhibition in cerebral cortex of immature rats following homocysteic acid-induced seizures. Exp Neurol 204:597–609. https://doi.org/10.1016/j.expneurol.2006.12.010
Folbergrová J, Ješina P, Haugvicová R, Lisý V, Houštěk J (2010) Sustained deficiency of mitochondrial complex I activity during long periods of survival after seizures induced in immature rats by homocysteic acid. Neurochem Int 56:394–403. https://doi.org/10.1016/j.neuint.2009.11.011
Folbergrová J, Otáhal J, Druga R (2012) Brain superoxide anion formation in immature rats during seizures: protection by selected compounds. Exp Neurol 233(1):421–429. https://doi.org/10.1016/j.expneurol.2011.11.009
Folbergrová J, Ješina P, Kubová H, Druga R, Otáhal J (2016) Status epilepticus in immature rats is associated with oxidative stress and mitochondrial dysfunction. Front Cell Neurosci 10:1–13. https://doi.org/10.3389/fncel.2016.00136
Folbergrová J (2016) Free radicals, oxidative stress, and epilepsy. In: Ahmad S (ed) Reactive oxygen species in biology and human health. CRC Press, Boca Raton, pp 147–153. https://doi.org/10.1201/b20228-15
Dobbing J (1970) Undernutrition and the developing brain. In: Himwich WA (ed) Developmental neurobiology. Thomas, Springfield, pp. 241–261
Hirsch E, Baram TZ, Snead OC (1992) Ontogenic study of lithium-pilocarpine-induced status epilepticus in rats. Brain Res 583(1-2):120–126. https://doi.org/10.1016/S0006-8993(10)80015-0
Bindokas VP, Jordán J, Lee CC, Miller RJ (1996) Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci 16(4):1324–1336
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic, New York
Liang LP, Ho YS, Patel M (2000) Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience 101(3):563–570. https://doi.org/10.1016/S0306-4522(00)00397-3
Ansari MA, Joshi G, Huang Q, Opii WO, Abdul HM, Sultana R, Butterfield DA (2006) In vivo administration of D609 leads to protection of subsequently isolated gerbil brain mitochondria subjected to in vitro oxidative stress induced by amyloid beta-peptide and other oxidative stressors: relevance to Alzheimer’s disease and other oxidative stress-related neurodegenerative disorders. Free Radic Biol Med 41(11):1694–1703. https://doi.org/10.1016/j.freeradbiomed.2006.09.002
Kubová H, Mareš P (2013) Are morphologic and functional consequences of status epilepticus in infant rats progressive? Neuroscience 235:232–249. https://doi.org/10.1016/j.neuroscience.2012.12.055
Waldbaum S, Liang LP, Patel M (2010) Persistent impairment of mitochondrial and tissue redox status during lithium-pilocarpine-induced epileptogenesis. J Neurochem 115(5):1172–1182. https://doi.org/10.1111/j.1471-4159.2010.07013.x
Williams S, Hamil N, Abramov AY, Walker MC, Kovac S (2015) Status epilepticus results in persistent overproduction of reactive oxygen species, inhibition of which is neuroprotective. Neuroscience 303:160–165. https://doi.org/10.1016/j.neuroscience.2015.07.005
Zielonka J, Kalyanaraman B (2010) Hydroethidine- and MitoSox-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth. Free Radic Biol Med 48(8):983–1001. https://doi.org/10.1016/j.freeradbiomed.2010.01.028
Kalyanaraman B, Dranka BP, Hardy M, Michalski R, Zielonka J (2014) HPLC-based monitoring of products formed from hydroethidine-based fluorogenic probes-the ultimate approach for intra- and extracellular superoxide detection. Biochim Biophys Acta 1840(2):739–744. https://doi.org/10.1016/j.bbagen.2013.05.008
Folbergrová J, Ješina P, Nůsková H, Houštěk J (2013) Antioxidant enzymes in cerebral cortex of immature rats following experimentally-induced seizures: Upregulation of mitochondrial MnSOD (SOD2). Int J Dev Neurosci 31(2):123–130. https://doi.org/10.1016/j.ijdevneu.2012.11.011
Sheldon RA, Jiang X, Francisco C, Christen S, Vexler ZS, Täuber MG, Ferriero DM (2004) Manipulation of antioxidant pathways in neonatal murine brain. Pediatric Res 56(4):656–662. https://doi.org/10.1203/01.PDR.00001.39413.27864.50
Ryan K, Liang LP, Rivard C, Patel M (2014) Temporal and spatial increase of reactive nitrogen species in the kainate model of temporal lobe epilepsy. Neurobiol Dis 64:8–15. https://doi.org/10.1016/j.nbd.2013.12.006
Ong W-Y, Liu X-R, Hu C-Y, Halliwell B (2000) Distribution of hydroxynonenal-modified proteins in the kainate-lesioned rat hippocampus: evidence that hydroxynonenal formation precedes neuronal cell death. Free Radic Biol Med 28(8):1214–1221. https://doi.org/10.1016/S0891-5849(00)00238-0
Bautista J, Corpas R, Ramos R, Cremades O, Gutiérrez JF, Alegre S (2000) Brain mitochondrial complex I inactivation by oxidative modification. Biochem Biophys Res Commun 275(3):890–894. https://doi.org/10.1006/bbrc.2000.3388
Brown GC, Borutaite V (2004) Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta 1658(1-2):44–49. https://doi.org/10.1016/j.bbabio.2004.03.016
Murray J, Taylor SW, Zhang B, Ghosh SS, Capaldi RA (2003) Oxidative damage to mitochondrial complex I due to peroxynitrite. J Biol Chem 278(39):37223–37230. https://doi.org/10.1074/jbc.M305694200
Pearce LL, Kanai AJ, Epperly MW, Peterson J (2005) Nitrosative stress results in irreversible inhibition of purified mitochondrial complexes I and III without modification of cofactors. Nitric Oxide 13(4):254–263. https://doi.org/10.1016/j.niox.2005.07.010
Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS (2006) Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J 394(3):627–634. https://doi.org/10.1042/BJ2005.1435
Dahm CC, Moore K, Murphy MP (2006) Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite. Implications for the interaction of nitric oxide with mitochondria. J Biol Chem 281:10056–10065. https://doi.org/10.1074/jbc.M512203200.
Ryan K, Backos DS, Reigan P, Patel M (2012) Post-translational oxidative modification and inactivation of mitochondrial complex I in epileptogenesis. J Neurosci 32(33):11250–11258. https://doi.org/10.1523/JNEUROSCI.0907-12.2012
DiMauro S, Hirano M (2005) Mitochondrial encephalomyopathies: an update. Neuromuscul Disord 15(4):276–286. https://doi.org/10.1016/j.nmd.2004.12.008
Robinson BH (1998) Human complex I deficiency: clinical spectrum and involvement of oxygen free radicals in the pathogenicity of the effect. Biochim Biophys Acta 1364:271–286
Kunz WS, Kudin AP, Vielhaber S, Blümcke, Zuschratter W, Schramm J, Beck H, Elger CE (2000) Mitochondrial complex I deficiency in the epileptic focus of patients with temporal lobe epilepsy. Ann Neurol 48:766–773. https://doi.org/10.1002/1531-8249(200011)48:5
Kudin AP, Kudina TA, Seyfried J, Vielhaber S, Beck H, Elger CE, Kunz WS (2002) Seizure-dependent modulation of mitochondrial oxidative phosphorylation in rat hippocampus. Eur J Neurosci 15(7):1105–1114. https://doi.org/10.1046/j.1460-9568.2002.01947.x
Chuang YC, Chang AYW, Lin J-W, Hsu SP, Chan SHH (2004) Mitochondrial dysfunction and ultrastructural damage in the hippocampus during kainic acid-induced status epilepticus in the rat. Epilepsia 45(10):1202–1209. https://doi.org/10.1111/j.0013-9580.2004.18204.x
Sipos I, Tretter L, Adam-Vizi V (2003) Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals. J Neurochem 84:112–118. https://doi.org/10.1046/j.1471-4159.2003.01513.x.
Kudin AP, Bimpong-Buta NYB, Vielhaber S, Elger CE, Kunz WS (2004) Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem 279(6):4127–4135. https://doi.org/10.1074/jbc.M310341200
Kussmaul L, Hirst J (2006) The mechanism of superoxide production by NADH:Ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A 103(20):7607–7612. https://doi.org/10.1073/pnas.0510977103
Fato R, Bergamini C, Leoni S, Strocchi P, Lenaz G (2008) Generation of reactive oxygen species by mitochondrial complex I: Implications in neurodegeneration. Neurochem Res 33:2487–2501. https://doi.org/10.1007/s11064-008-9747-0.
Perier C, Tieu K, Guégan C, Caspersen C, Jackson-Lewis V, Carelli V, Martinuzzi A, Hirano M et al (2005) Complex I deficiency primes Bax-dependent neuronal aptosis through mitochondrial oxidative damage. Proc Natl Acad Sci U S A 102(52):19126–19131. https://doi.org/10.1073/pnas.0508215102
Marella M, Seo BB, Matsuno-Yagi A, Yagi T (2007) Mechanism of cell death caused by complex I defects in a rat dopaminergic cell line. J Biol Chem 282(33):24146–24156. https://doi.org/10.1074/jbc.M701819200
Yamakura F, Taka H, Fujimura T, Murayama K (1998) Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem 273(23):14085–14089. https://doi.org/10.1074/jbc.273.23.14085
MacMillan-Crow LA, Crow JP, Thompson JA (1998) Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry 37(6):1613–1622. https://doi.org/10.1021/bi971894b
Bayir H, Kagan VE, Clark RSB, Janesko-Feldman K, Rafikov R, Huang Z, Zhang X, Vagni V et al (2007) Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J Neurochem 101(1):168–181. https://doi.org/10.1111/j.1471-4159.2006.04353.x
Bidmon HJ, Gorg B, Palomero-Gallacher N, Schleicher A, Haussinger D, Speckmann EJ, Zilles K (2008) Glutamine synthetase becomes nitrated and its activity reduced during repetitive seizure activity in the pentylentetrazole model of epilepsy. Epilepsia 49(10):1733–1748. https://doi.org/10.1111/j.1528-1167.2008.01642.x
Van der Hel WS, Hessel EVS, Bos IWM, Mulder S, Verlinde SAMW, van Eijsden P, de Graan PNE (2014) Persistent reduction of hippocampal glutamine synthetase expression after status epilepticus in immature rats. Eur J Neurosci 40(12):3711–3719. https://doi.org/10.1111/ejn.12756
Trotti D, Danbolt NC, Volterra A (1998) Glutamate transporters are oxidant-vulnerable: molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci 19(8):328–334. https://doi.org/10.1016/S0165-6147(98)01230-9
Kong Q, Takahashi K, Schulte D, Stouffer N, Lin Y, Lin CL (2012) Increased glial glutamate transporter EAAT2 expression reduces epileptogenic processes following pilocarpine-induced status epilepticus. Neurobiol Dis 47(2):145–154. https://doi.org/10.1016/j.nbd.2012.03.032
Sigleton RH, Yan HQ, Fellows-Mayle W, Dixon CE (2010) Resveratrol attenuates behavioral impairments and reduces cortical and hippocampal loss in a rat controlled cortical impact model of traumatic brain injury. J Neurotrauma 27(6):1091–1099. https://doi.org/10.1089/neu.2010.1291
Sakata Y, Zhuang H, Kwansa H, Koehler RC, Doré S (2010) Resveratrol protects against experimental stroke: Putative neuroprotective role of heme oxygenase 1. Exp Neurol 224(1):325–329. https://doi.org/10.1016/j.expneurol.2010.03.032
Kroon PA, Iyer A, Chunduri P, Chan V, Brown L (2010) The cardiovascular potential. Nutrapharmacology of resveratrol: pharmacokinetics, molecular mechanisms and therapeutic potential. Curr. Med Chem 17:2442–2455
Gupta YK, Briyal S, Chaudhary G (2002) Protective effect of trans-resveratrol against kainic acid-induced seizures and oxidative stress in rats. Pharmacol Biochem Behav 71:245–249. https://doi.org/10.1016/s0091-3057(01)00663-3.
Wu Z, Xu Q, Zhang L, Kong D, Ma R, Wang L (2009) Protective effect of resveratrol against kainate-induced temporal lobe epilepsy in rats. Neurochem Res 34(8):1393–1400. https://doi.org/10.1007/s11064-009-9920-0
Shetty AK (2011) Promise of resveratrol for easing status epilepticus and epilepsy. Pharmacol Ther 131(3):269–286. https://doi.org/10.1016/j.pharmthera2011.04.008
Wang S, Bo Q, Zhao X, Yang X, Chi Z, Liu X (2013) Resveratrol pre-treatment reduces early inflammatory responses induced by status epilepticus via mTOR signaling. Brain Res 1492:122–129. https://doi.org/10.1016/j.brainres.2012.11.027
Mishra V, Shuai B, Kodali M, Shetty GA, Hattiangady B, Rao X, Shetty AK (2015) Resveratrol treatment after status epilepticus restrains neurodegeneration and abnormal neurogenesis with suppression of oxidative stress and inflammation. Sci Rep 5(1):1–18. https://doi.org/10.1038/srep17807
Friedman LK, Goldstein B, Rafiuddin A, Roblejo P, Friedman S (2013) Lack of resveratrol neuroprotection in developing rats treated with kainic acid. Neuroscience 230:39–49. https://doi.org/10.1016/j.neuroscience.2012.10.063
Rong Y, Doctrow SR, Tocco G, Baudry M (1999) EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and atttenuates kainate-induced neuropathology. Proc Natl Acad Sci U S A 96(17):9897–9902. https://doi.org/10.1073/pnas.96.17.9897
Frantseva MV, Perez Velazquez JL, Tsoraklidis G, Mendonca AJ, Adamchik Y, Mills LR, Carlen PL, Burnham MW (2000) Oxidative stress is involved in seizure-induced neurodegeneration in the kindling model of epilepsy. Neuroscience 97(3):431–435. https://doi.org/10.1016/S0306-4522(00)00041-5
Tomaciello F, Leclercq K, Kaminski RM (2016) Resveratrol lacks protective activity against acute seizures in mouse models. Neurosci Lett 632:199–203. https://doi.org/10.1016/j.neulet.2016.09.002
Holthoff JH, Woodling KA, Doergr DR, Burns ST, Hinson JA, Mayeux PR (2010) Resveratrol, a dietary polyphenolic phytoalexin, is a functional scavenger of peroxynitrite. Biochem Pharmacol 80(8):1260–1265. https://doi.org/10.1016/j.bcp.2010.06.027
Folbergrová J, Ješina P, Kubová H, Otáhal J (2015) Resveratrol attenuates oxidative stress associated with status epilepticus in immature rats. Epilepsia 56(Suppl.1):3–263.P0469
Sahebkar A (2010) Neuroprotective effects of resveratrol: Potential mechanisms. Neurochem Int 57(6):621–622. https://doi.org/10.1016/j.neuint.2010.06.014
Kesherwani V, Atif F, Yousuf S, Agrawal SK (2013) Resveratrol protects spinal cord dorsal column from hypoxic injury by activating Nrf2. Neuroscience 241:80–88. https://doi.org/10.1016/j.neuroscience.2013.03.015
Narayanan SV, Dave KR, Saul I, Perez-Pinzon MA (2015) Resveratrol preconditioning protects against cerebral ischemic injury via nuclear erythroid 2-related factor 2. Stroke 46(6):1626–1632. https://doi.org/10.1161/STROKEAHA.115.008921
Mazzuferi M, Kumar G, van Eyll J, Danis B, Foerch P, Kaminski RM (2013) Nrf2 defense pathway:Experimental evidence for its protective role in epilepsy. Ann Neurol 74(4):560–568. https://doi.org/10.1002/ana.23940
Wang W, Wu YF, Zhang GL, Fang HB, Wang HC, Zang HM, Xie T, Wang WP (2014) Activation of Nrf2-ARE signal pathway protects the brain from damage induced by epileptic seizure. Brain Res 1544:54–61. https://doi.org/10.1016/j.brainres.2013.12.004
Pauletti A, Terrone G, Shekh-Ahmad T, Salamone A, Ravizza T, Rizzi M, Pastore A, Pascente R et al (2017) Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain 140(7):1885–1899. https://doi.org/10.1093/brain/awx117
Acknowledgments
This work was supported by grants #P303/10/0999 and #15-08565S from the Czech Science Foundation and with institutional support RVO: 6798523. The authors express their thanks to E. Lažková and V. Brožková for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Folbergrová, J., Ješina, P., Kubová, H. et al. Effect of Resveratrol on Oxidative Stress and Mitochondrial Dysfunction in Immature Brain during Epileptogenesis. Mol Neurobiol 55, 7512–7522 (2018). https://doi.org/10.1007/s12035-018-0924-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0924-0