Abstract
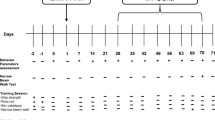
Neurotrophic factors (NTFs) are a promising therapeutic option for Parkinson’s disease (PD). They exert their function through tyrosine kinase receptors. Our goal was to assess the effects of administering a selective tyrosine kinase inhibitor (vandetanib) that blocks VEGFR2 and RET receptors in a preclinical model of PD. Rats underwent intrastriatal injections of 6-hydroxydopamine (6-OHDA). Two weeks later, the rats received 30 mg/kg vandetanib or saline orally. The effects were assessed using the rotational behavioral test, tyrosine hydroxylase (TH) immunohistochemistry, and western blot. In 6-OHDA-lesioned rats, motor symptoms were almost undetectable, but morphological and biochemical changes were significant. Vandetanib treatment, combined with the presence of 6-OHDA lesions, significantly increased behavioral impairment and morphological and biochemical changes. Therefore, after vandetanib treatment, the TH-immunopositive striatal volume, the percentage of TH+ neurons, and the extent of the axodendritic network in the substantia nigra decreased. Glial fibrillary acidic protein-positivity significantly decreased in the striatum and substantia nigra in the vandetanib-treated group. In addition, p-Akt and p-ERK 1/2 levels were significantly lower and caspase-3 expression significantly increased after vandetanib administration. In conclusion, we demonstrate for the first time the deleterious effect of a tyrosine kinase inhibitor on the dopaminergic system, supporting the beneficial and synergistic effect of NTFs reported in previous papers.






Similar content being viewed by others
Change history
05 March 2018
The authors found a terrible mistake in the manuscript. The legends from the Fig. 5 and 6 are interchanged. The Fig. 5 should be appeared with the legend from the Fig. 6 and Fig. 6 should be appeared with the legend from the Fig. 5.
References
Hirsch EC, Jenner P, Przedborski S (2013) Pathogenesis of Parkinson’s disease. Mov Disord 28:24–30. doi:10.1002/mds.25032
Ramaswamy S, Kordower JH (2009) Are growth factors the answer? Parkinsonism Relat Disord 15(Suppl 3):S176–S180. doi:10.1016/S1353-8020(09)70809-0
Yasuda T, Mochizuki H (2010) Use of growth factors for the treatment of Parkinson’s disease. Expert Rev Neurother 10:915–924. doi:10.1586/ern.10.55
Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383–394. doi:10.1038/nrn812
Kramer ER, Aron L, Ramakers GMJ et al (2007) Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol 5:e39. doi:10.1371/journal.pbio.0050039
Drinkut A, Tillack K, Meka DP et al (2016) Ret is essential to mediate GDNF’s neuroprotective and neuroregenerative effect in a Parkinson disease mouse model. Cell Death Dis 7:e2359. doi:10.1038/cddis.2016.263
Ferrara N (2009) Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol 29:789–791. doi:10.1161/ATVBAHA.108.179663
Shibuya M (2011) Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2:1097–1105. doi:10.1177/1947601911423031
Musumeci F, Radi M, Brullo C, Schenone S (2012) Vascular endothelial growth factor (VEGF) receptors: drugs and new inhibitors. J Med Chem 55:10797–10822. doi:10.1021/jm301085w
Storkebaum E, Lambrechts D, Carmeliet P (2004) VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. BioEssays 26:943–954. doi:10.1002/bies.20092
Lafuente JV, Argandoña EG, Mitre B (2006) VEGFR-2 expression in brain injury: Its distribution related to brain-blood barrier markers. J Neural Transm 113:487–496. doi:10.1007/s00702-005-0407-0
Wick A, Wick W, Waltenberger J et al (2002) Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J Neurosci 22:6401–6407
Kaya D, Gürsoy-Ozdemir Y, Yemisci M et al (2005) VEGF protects brain against focal ischemia without increasing blood-brain permeability when administered intracerebroventricularly. J Cereb Blood Flow Metab 25:1111–1118. doi:10.1038/sj.jcbfm.9600109
Yasuhara T, Shingo T, Kobayashi K et al (2004) Neuroprotective effects of vascular endothelial growth factor (VEGF) upon dopaminergic neurons in a rat model of Parkinson’s disease. Eur J Neurosci 19:1494–1504. doi:10.1111/j.1460-9568.2004.03254.x
Yasuhara T, Shingo T, Muraoka K et al (2005) Neurorescue effects of VEGF on a rat model of Parkinson’s disease. Brain Res 1053:10–18. doi:10.1016/j.brainres.2005.05.027
Herrán E, Ruiz-Ortega JÁ, Aristieta A et al (2013) In vivo administration of VEGF- and GDNF-releasing biodegradable polymeric microspheres in a severe lesion model of Parkinson’s disease. Eur J Pharm Biopharm 85:1183–1190. doi:10.1016/j.ejpb.2013.03.034
Requejo C, Ruiz-Ortega JA, Bengoetxea H et al (2015) Topographical distribution of morphological changes in a partial model of Parkinson’s disease-effects of nanoencapsulated neurotrophic factors administration. Mol Neurobiol. doi:10.1007/s12035-015-9234-y
Herrán E, Requejo C, Ruiz-Ortega JA et al (2014) Increased antiparkinson efficacy of the combined administration of VEGF- and GDNF-loaded nanospheres in a partial lesion model of Parkinson’s disease. Int J Nanomedicine 9:2677–2687. doi:10.2147/IJN.S61940
Requejo C, Ruiz-Ortega JA, Bengoetxea H et al (2016) Morphological changes in a severe model of Parkinson’s disease and its suitability to test the therapeutic effects of microencapsulated neurotrophic factors. Mol Neurobiol 1–14. doi: 10.1007/s12035-016-0244-1
Knight ZA, Lin H, Shokat KM (2010) Targeting the cancer kinome through polypharmacology. Nat Rev Cancer 10:130–137. doi:10.1038/nrc2787
Lorusso PM, Eder JP (2008) Therapeutic potential of novel selective-spectrum kinase inhibitors in oncology. Expert Opin Investig Drugs 17:1013–1028. doi:10.1517/13543784.17.7.1013
Huo Z, Yu S, Hong S et al (2016) A systematic review and meta-analysis of the risk of diarrhea associated with vandetanib treatment in carcinoma patients. Onco Targets Ther 9:3621–3631. doi:10.2147/OTT.S96830
Paxinos G, Watson C (2013) The rat brain in stereotaxic coordinates Hard Cover Edition. Academic Press
Argandoña EG, Bengoetxea H, Bulnes S et al (2012) Effect of intracortical vascular endothelial growth factor infusion and blockade during the critical period in the rat visual cortex. Brain Res 1473:141–154. doi:10.1016/j.brainres.2012.07.008
Yue X, Hariri DJ, Caballero B et al (2014) Comparative study of the neurotrophic effects elicited by VEGF-B and GDNF in preclinical in vivo models of Parkinson’s disease. Neuroscience 258:385–400. doi:10.1016/j.neuroscience.2013.11.038
Kirik D, Rosenblad C, Björklund A (2000) Preservation of a functional nigrostriatal dopamine pathway by GDNF in the intrastriatal 6-OHDA lesion model depends on the site of administration of the trophic factor. Eur J Neurosci 12:3871–3882
Nakajima K, Hida H, Shimano Y et al (2001) GDNF is a major component of trophic activity in DA-depleted striatum for survival and neurite extension of DAergic neurons. Brain Res 916:76–84. doi:10.1016/S0006-8993(01)02866-9
Kramer ER, Liss B (2015) GDNF-Ret signaling in midbrain dopaminergic neurons and its implication for Parkinson disease. FEBS Lett 589:3760–3772. doi:10.1016/j.febslet.2015.11.006
Stott SRW, Barker RA (2014) Time course of dopamine neuron loss and glial response in the 6-OHDA striatal mouse model of Parkinson’s disease. Eur J Neurosci 39:1042–1056. doi:10.1111/ejn.12459
Hernandez-Baltazar D, Mendoza-Garrido ME, Martinez-Fong D (2013) Activation of GSK-3β and caspase-3 occurs in nigral dopamine neurons during the development of apoptosis activated by a striatal injection of 6-hydroxydopamine. PLoS One 8:1–13. doi:10.1371/journal.pone.0070951
Kunnimalaiyaan M, Ndiaye M, Chen H (2006) Apoptosis-mediated medullary thyroid cancer growth suppression by the PI3K inhibitor LY294002. Surgery 140:1009-14–1009-15. doi:10.1016/j.surg.2006.06.040
Spanheimer PM, Cyr AR, Gillum MP et al (2014) Distinct pathways regulated by RET and estrogen receptor in luminal breast cancer demonstrate the biological basis for combination therapy. Ann Surg 259:793–799. doi:10.1097/SLA.0b013e3182a6f552
Nicole O, Ali C, Docagne F et al (2001) Neuroprotection mediated by glial cell line-derived neurotrophic factor: involvement of a reduction of NMDA-induced calcium influx by the mitogen-activated protein kinase pathway. J Neurosci 21
Karakaya S, Kipp M, Beyer C (2007) Oestrogen regulates the expression and function of dopamine transporters in astrocytes of the nigrostriatal system. J Neuroendocrinol 19:682–690. doi:10.1111/j.1365-2826.2007.01575.x
Timmons S, Coakley MF, Moloney AM, O’Neill C (2009) Akt signal transduction dysfunction in Parkinson’s disease. Neurosci Lett 467:30–35. doi:10.1016/j.neulet.2009.09.055
Cui W, Li W, Han R et al (2011) PI3-K/Akt and ERK pathways activated by VEGF play opposite roles in MPP+-induced neuronal apoptosis. Neurochem Int 59:945–953. doi:10.1016/j.neuint.2011.07.005
Francardo V, Bez F, Wieloch T et al Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. doi: 10.1093/brain/awu107
Azkona G, Sagarduy A, Aristieta A et al (2014) Buspirone anti-dyskinetic effect is correlated with temporal normalization of dysregulated striatal DRD1 signalling in L-DOPA-treated rats. Neuropharmacology 79:726–737. doi:10.1016/j.neuropharm.2013.11.024
Fuqua JL, Littrell OM, Lundblad M et al (2014) Dynamic changes in dopamine neuron function after DNSP-11 treatment: effects in vivo and increased ERK 1/2 phosphorylation in vitro. Peptides 54:1–8. doi:10.1016/j.peptides.2013.12.007
Lindgren N, Francardo V, Quintino L et al (2012) A model of GDNF gene therapy in mice with 6-hydroxydopamine lesions: time course of neurorestorative effects and ERK1/2 activation. J Parkinsons Dis 2:333–348. doi:10.3233/JPD-012146
Paulus W, Jellinger K (1991) The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol 50:743–755
Morales I, Sanchez A, Rodriguez-Sabate C, Rodriguez M (2016) The astrocytic response to the dopaminergic denervation of the striatum. J Neurochem:81–95. doi:10.1111/jnc.13684
Rossi D (2015) Astrocyte physiopathology: at the crossroads of intercellular networking, inflammation and cell death. Prog Neurobiol 130:86–120. doi:10.1016/j.pneurobio.2015.04.003
Halliday GM, Stevens CH (2011) Glia: initiators and progressors of pathology in Parkinson’s disease. Mov Disord 26:6–17. doi:10.1002/mds.23455
Pellegrini E, Diotel N, Vaillant-Capitaine C et al (2016) Steroid modulation of neurogenesis: focus on radial glial cells in zebrafish. J Steroid Biochem Mol Biol 160:27–36. doi:10.1016/j.jsbmb.2015.06.011
Episcopo FL, Tirolo C, Testa N et al (2013) Reactive astrocytes are key players in nigrostriatal dopaminergic neurorepair in the MPTP mouse model of Parkinson’s disease: focus on endogenous neurorestoration. Curr Aging Sci 6:45–55
Ben Haim L, Carrillo-de Sauvage M-A, Ceyzériat K, Escartin C (2015) Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci 9:278. doi:10.3389/fncel.2015.00278
Chen P-S, Peng G-S, Li G et al (2006) Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry 11:1116–1125. doi:10.1038/sj.mp.4001893
Tian Y, Tang C-J, Wang J et al (2007) Favorable effects of VEGF gene transfer on a rat model of Parkinson disease using adeno-associated viral vectors. Neurosci Lett 421:239–244. doi:10.1016/j.neulet.2007.05.033
Acknowledgments
The authors appreciate the support from the University of the Basque Country (UPV/EHU) (UFI 11/32), the Basque Government (GIC IT 901/16 and 747-13, PPG 17/51), “Ministerio de Ciencia e Innovación” SAF 2016-77758-R (AEI/FEDER, UE), and SGIker (UPV/EHU). C. Requejo appreciates the UPV/EHU for a fellowship subvention.
Author information
Authors and Affiliations
Corresponding author
Additional information
A correction to this article is available online at https://doi.org/10.1007/s12035-018-0979-y.
Rights and permissions
About this article
Cite this article
Requejo, C., Ruiz-Ortega, J.A., Bengoetxea, H. et al. Deleterious Effects of VEGFR2 and RET Inhibition in a Preclinical Model of Parkinson’s Disease. Mol Neurobiol 55, 201–212 (2018). https://doi.org/10.1007/s12035-017-0733-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0733-x