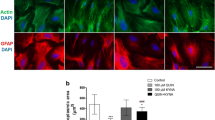
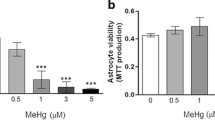
Abstract
Cytoskeletal proteins are increasingly recognized as having important roles as a target of the action of different neurotoxins. In the last years, several works of our group have shown that quinolinic acid (QUIN) was able to disrupt the homeostasis of the cytoskeleton of neural cells and this was associated with cell dysfunction and neurodegeneration. QUIN is an excitotoxic metabolite of tryptophan metabolism and its accumulation is associated with several neurodegenerative diseases. In the present review, we provide a comprehensive view of the actions of QUIN upstream of glutamate receptors, eliciting kinase/phosphatase signaling cascades that disrupt the homeostasis of the phosphorylation system associated with intermediate filament proteins of astrocytes and neurons. We emphasize the critical role of calcium in these actions and the evidence that misregulated cytoskeleton takes part of the cell response to the injury resulting in neurodegeneration in different brain regions, disrupted cell signaling in acute tissue slices, and disorganized cytoskeleton with altered cell morphology in primary cultures. We also discuss the interplay among misregulated cytoskeleton, oxidative stress, and cell-cell contact through gap junctions mediating the quinolinic acid injury in rat brain. The increasing amount of cross talks identified between cytoskeletal proteins and cellular signaling cascades reinforces the exciting possibility that cytoskeleton could be a new target in the neurotoxicity of QUIN and further studies will be necessary to develop strategies to protect the cytoskeleton and counteracts the cytotoxicity of this metabolite.



Similar content being viewed by others
References
Chen Y, Guillemin GJ (2009) Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res 2:1–19
Schwarcz R, Bruno JP, Muchowski PJ, Wu H-Q (2012) Kynurenines in the mammalian brain: when physiology meets pathology. Nature Reviews/Neuroscience 13:464–476
Lugo-Huitron R, Ugalde Muniz P, Pineda B, Pedraza-Chaverri J, Rios C, Perez-de la Cruz V (2013) Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxidative Med Cell Longev 2013:104024. doi:10.1155/2013/104024
Perez-De La Cruz V, Carrillo-Mora P, Santamaria A (2012) Quinolinic acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. Int J Tryptophan Res 5:1–8. doi:10.4137/IJTR.S8158
Pessoa-Pureur R, Wajner M (2007) Cytoskeleton as a potential target in the neuropathology of maple syrup urine disease: insight from animal studies. J Inherit Metab Dis Oct;30(5)::664–672
Zamoner A, and Pessoa-Pureur R (2011) Nongenomic actions of thyroid hormones: every why has a wherefore. Immunology, Endocrine & Metabolic Agents in medical chemistry 11(3): :165-178
Pessoa-Pureur R, Heimfarth L, Rocha JB (2014) Signaling mechanisms and disrupted cytoskeleton in the diphenyl ditelluride neurotoxicity. Oxidative Med Cell Longev 2014:458601. doi:10.1155/2014/458601
Huber F, Boire A, Lopez MP, Koenderink GH (2015) Cytoskeletal crosstalk: when three different personalities team up. Curr Opin Cell Biol 32:39–47. doi:10.1016/j.ceb.2014.10.005
Bolin K, Rachmaninoff N, Moncada K, Pula K, Kennell J, Buttitta L (2016) miR-8 modulates cytoskeletal regulators to influence cell survival and epithelial organization in drosophila wings. Dev Biol 412(1):83–98. doi:10.1016/j.ydbio.2016.01.041
Yi B, Chen L, Zeng J, Cui J, Wang G, Qian G, Belguise K, Wang X, Lu K (2015) Ezrin regulating the cytoskeleton remodeling is required for hypoxia-induced myofibroblast proliferation and migration. Front Cardiovasc Med 3: 2:10,
Compagnucci C, Piemonte F, Sferra A, Piermarini E, Bertini E (2016) The cytoskeletal arrangements necessary to neurogenesis. Oncotarget 7(15):19414–19429. doi:10.18632/oncotarget.6838
Le Clainche C, Carlier MF (2008) Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev 88(2):489–513. doi:10.1152/physrev.00021.2007
Xiong T, Liu J, Dai G, Hou Y, Tan B, Zhang Y, Li S, Song Y et al (2015) The progressive changes of filamentous actin cytoskeleton in the hippocampal neurons after pilocarpine-induced status epilepticus. Epilepsy Res 118:55–67. doi:10.1016/j.eplepsyres.2015.11.002
Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U (2007) Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol 8(7):562–573. doi:10.1038/nrm2197
Gentil BJ, Tibshirani M, Durham HD (2015) Neurofilament dynamics and involvement in neurological disorders. Cell Tissue Res 360(3):609–620. doi:10.1007/s00441-014-2082-7
Laser-Azogui A, Kornreich M, Malka-Gibor E, Beck R (2015) Neurofilament assembly and function during neuronal development. Curr Opin Cell Biol 32:92–101. doi:10.1016/j.ceb.2015.01.003
Beck R, Deek J, Choi MC, Ikawa T, Watanabe O, Frey E, Pincus P, Safinya CR (2010) Unconventional salt trend from soft to stiff in single neurofilament biopolymers. Langmuir 26(24):18595–18599. doi:10.1021/la103655x
Mellad JA, Warren DT, Shanahan CM (2011) Nesprins LINC the nucleus and cytoskeleton. Curr Opin Cell Biol 23(1):47–54. doi:10.1016/j.ceb.2010.11.006
Rao MV, Engle LJ, Mohan PS, Yuan A, Qiu D, Cataldo A, Hassinger L, Jacobsen S et al (2002) Myosin Va binding to neurofilaments is essential for correct myosin Va distribution and transport and neurofilament density. J Cell Biol 159(2):279–290. doi:10.1083/jcb.200205062
Rao MV, Mohan PS, Kumar A, Yuan A, Montagna L, Campbell J, Veeranna EEM, Julien JP et al (2011) The myosin Va head domain binds to the neurofilament-L rod and modulates endoplasmic reticulum (ER) content and distribution within axons. PLoS One 6(2):e17087. doi:10.1371/journal.pone.0017087
Macioce P, Gandolfi N, Leung CL, Chin SS, Malchiodi-Albedi F, Ceccarini M, Petrucci TC, Liem RK (1999) Characterization of NF-L and betaIISigma1-spectrin interaction in live cells. Exp Cell Res 250(1):142–154. doi:10.1006/excr.1999.4479
Wiche G, Winter L (2011) Plectin isoforms as organizers of intermediate filament cytoarchitecture. BioArchitecture 1(1):14–20. doi:10.4161/bioa.1.1.14630
Koutras C, Levesque G (2011) Identification of novel NPRAP/delta-catenin-interacting proteins and the direct association of NPRAP with dynamin 2. PLoS One 6(10):e25379. doi:10.1371/journal.pone.0025379
Ehlers MD, Fung ET, O'Brien RJ, Huganir RL (1998) Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J Neurosci 18(2):720–730
Yabe JT, Chylinski T, Wang FS, Pimenta A, Kattar SD, Linsley MD, Chan WK, Shea TB (2001) Neurofilaments consist of distinct populations that can be distinguished by C-terminal phosphorylation, bundling, and axonal transport rate in growing axonal neurites. J Neurosci 21(7):2195–2205
Volterra A, Meldolesi J (2005) Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6(8):626–640. doi:10.1038/nrn1722
Pirttimaki TM, Parri HR (2013) Astrocyte plasticity: implications for synaptic and neuronal activity. Neuroscientist 19(6):604–615. doi:10.1177/1073858413504999
Middeldorp J, Hol EM (2011) GFAP in health and disease. Prog Neurobiol 93(3):421–443. doi:10.1016/j.pneurobio.2011.01.005
Hol EM, Pekny M (2015) Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol 32:121–130. doi:10.1016/j.ceb.2015.02.004
Orre M, Kamphuis W, Osborn LM, Jansen AH, Kooijman L, Bossers K, Hol EM (2014) Isolation of glia from Alzheimer’s mice reveals inflammation and dysfunction. Neurobiol Aging 35(12):2746–2760. doi:10.1016/j.neurobiolaging.2014.06.004
Kamphuis W, Orre M, Kooijman L, Dahmen M, Hol EM (2012) Differential cell proliferation in the cortex of the APPswePS1dE9 Alzheimer’s disease mouse model. Glia 60(4):615–629. doi:10.1002/glia.22295
Kamphuis W, Middeldorp J, Kooijman L, Sluijs JA, Kooi EJ, Moeton M, Freriks M, Mizee MR et al (2014) Glial fibrillary acidic protein isoform expression in plaque related astrogliosis in Alzheimer’s disease. Neurobiol Aging 35(3):492–510. doi:10.1016/j.neurobiolaging.2013.09.035
Ubersax JA, Ferrell JE Jr (2007) Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol 8(7):530–541. doi:10.1038/nrm2203
Sihag RK, Nixon RA (1991) Identification of Ser-55 as a major protein kinase a phosphorylation site on the 70-kDa subunit of neurofilaments. Early turnover during axonal transport. J Biol Chem 266(28):18861–18867
Omary MB, Ku NO, Tao GZ, Toivola DM, Liao J (2006) "heads and tails" of intermediate filament phosphorylation: multiple sites and functional insights. Trends Biochem Sci 31(7):383–394. doi:10.1016/j.tibs.2006.05.008
Shea TB, Chan WK (2008) Regulation of neurofilament dynamics by phosphorylation. Eur J Neurosci 27(8):1893–1901. doi:10.1111/j.1460-9568.2008.06165.x
Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant HC (2007) Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp Cell Res 313(10):2098–2109. doi:10.1016/j.yexcr.2007.04.010
Zhou J, Wang H, Feng Y, Chen J (2010) Increased expression of cdk5/p25 in N2a cells leads to hyperphosphorylation and impaired axonal transport of neurofilament proteins. Life Sci 86:532–537
GRANT P, PANT HC (2000) Neurofilament protein synthesis and phosphorylation. Journal of Neurocytology 29:843–872
Lee S, Pant HC, Shea TB (2014) Divergent and convergent roles for kinases and phosphatases in neurofilament dynamics. J Cell Sci 127(Pt 18):4064–4077. doi:10.1242/jcs.153346
Lewis SE, Nixon RA (1988) Multiple phosphorylated variants of the high molecular mass subunit of neurofilaments in axons of retinal cell neurons: characterization and evidence for their differential association with stationary and moving neurofilaments. J Cell Biol 107(6 Pt 2):2689–2701
Yabe JT, Pimenta A, Shea TB (1999) Kinesin-mediated transport of neurofilament protein oligomers in growing axons. J Cell Sci 112 ( Pt 21):3799–3814
Shea TB, Chan WK, Kushkuley J, Lee S (2009) Organizational dynamics, functions, and pathobiological dysfunctions of neurofilaments. Results Probl Cell Differ 48:29–45. doi:10.1007/400_2009_8
Motil J, Chan WK, Dubey M, Chaudhury P, Pimenta A, Chylinski TM, Ortiz DT, Shea TB (2006) Dynein mediates retrograde neurofilament transport within axons and anterograde delivery of NFs from perikarya into axons: regulation by multiple phosphorylation events. Cell Motil Cytoskeleton 63(5):266–286. doi:10.1002/cm.20122
Shea TB, Chan A (2008) S-adenosyl methionine: a natural therapeutic agent effective against multiple hallmarks and risk factors associated with Alzheimer’s disease. J Alzheimers Dis 13(1):67–70
Bajaj NPS, Al-Sarraj ST, Leigh PN, Anderson V, Miller CCJ (1999) Cyclin dependent kinase 5 (cdk5) phosphorylates neurofilament heavy (NF-H) chain to generate epitopes for antibodies that label neurofilament affected motor neurons in ALS. Neuro-Psychopharm Biol Psychiat 23:833–850
Strong MJ, Strong WL, Jaffe H, Traggert B, Sopper MM, Pant HC (2001) Phosphorylation state of the native high-molecular-weight neurofilament subunit protein from cervical spinal cord in sporadic amyotrophic lateral sclerosis. J Neurochem 76(5):1315–1325
Sontag E, Hladik C, Montgomery L, Luangpirom A, Mudrak I, Ogris E, White CL 3rd (2004) Downregulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis. J Neuropathol Exp Neurol 63(10):1080–1091
Loureiro SO, Heimfarth L, Lacerda BA, Vidal LF, Soska A, dos Santos NG, de Souza Wyse AT, Pessoa-Pureur R (2010) Homocysteine induces hypophosphorylation of intermediate filaments and reorganization of actin cytoskeleton in C6 glioma cells. Cell Mol Neurobiol 30(4):557–568. doi:10.1007/s10571-009-9480-5
Fernandes CG, Pierozan P, Soares GM, Ferreira F, Zanatta A, Amaral AU, Borges CG, Wajner M et al (2015) NMDA receptors and oxidative stress induced by the major metabolites accumulating in HMG Lyase deficiency mediate hypophosphorylation of cytoskeletal proteins in brain from adolescent rats: potential mechanisms contributing to the neuropathology of this disease. Neurotox Res 28(3):239–252. doi:10.1007/s12640-015-9542-z
Carvalho RV, da Silva FF, Heimfarth L, Pierozan P, Fernandes C, Pessoa-Pureur R (2016) Acute hyperammonemia induces NMDA-mediated hypophosphorylation of intermediate filaments through PP1 and PP2B in cerebral cortex of young rats. Neurotox Res 30(2):138–149. doi:10.1007/s12640-016-9607-7
Bordelon YM, Chesselet MF, Nelson D, Welsh F, Erecinska M (1997) Energetic dysfunction in quinolinic acid-lesioned rat striatum. J Neurochem 69(4):1629–1639
Portera-Cailliau C, Hedreen JC, Price DL, Koliatsos VE (1995) Evidence for apoptotic cell death in Huntington disease and excitotoxic animal models. J Neurosci 15 (5 Pt 2):3775–3787
Braidy N, Grant R, Adams S, Brew BJ, Guillemin GJ (2009) Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotox Res 16(1):77–86. doi:10.1007/s12640-009-9051-z
Ramaswamy S, McBride JL, Kordower JH (2007) Animal models of Huntington’s disease. ILAR J 48(4):356–373
Pierozan P, Zamoner A, Soska AK, Silvestrin RB, Loureiro SO, Heimfarth L, Mello e Souza T, Wajner M, Pessoa-Pureur R (2010) Acute intrastriatal administration of quinolinic acid provokes hyperphosphorylation of cytoskeletal intermediate filament proteins in astrocytes and neurons of rats. Exp Neurol 224 (1):188–196. doi:10.1016/j.expneurol.2010.03.009
Gill SR, Wong PC, Monteiro MJ, Cleveland DW (1990) Assembly properties of dominant and recessive mutations in the small mouse neurofilament (NF-L) subunit. J Cell Biol 111(5 Pt 1):2005–2019
Heins S, Wong PC, Muller S, Goldie K, Cleveland DW, Aebi U (1993) The rod domain of NF-L determines neurofilament architecture, whereas the end domains specify filament assembly and network formation. J cell Biol 123(6 Pt 1):1517–1533
Pierozan P, Gonçalves FC, Ferreira F, Pessoa-Pureur R (2014) Acute intrastriatal injection of quinolinic acid provokes long-lasting misregulation of the cytoskeleton in the striatum, cerebral cortex and hippocampus of young rats. Brain res Aug 19:1577:1571–1510
Holmgren A, Bouhy D, Timmerman V (2012) Neurofilament phosphorylation and their proline-directed kinases in health and disease. J Peripher Nerv Syst 17(4):365–376. doi:10.1111/j.1529-8027.2012.00434.x
Pierozan P, Fernandes CG, Dutra MF, Pandolfo P, Ferreira F, de Lima BO, Porciuncula L, Wajner M et al (2014) Biochemical, histopathological and behavioral alterations caused by intrastriatal administration of quinolic acid to young rats. FEBS J 281(8):2061–2073. doi:10.1111/febs.12762
Pierozan P, Zamoner A, Soska AK, de Lima BO, Reis KP, Zamboni F, Wajner M, Pessoa-Pureur R (2012) Signaling mechanisms downstream of quinolinic acid targeting the cytoskeleton of rat striatal neurons and astrocytes. Exp Neurol 233(1):391–399. doi:10.1016/j.expneurol.2011.11.005
Steiner D, Saya D, Schallmach E, Simonds WF, Vogel Z (2006) Adenylyl cyclase type-VIII activity is regulated by G (betagamma) subunits. Cell Signal 18(1):62–68
Bonsi P, Platania P, Martella G, Madeo G, Vita D, Tassone A, Bernardi G, Pisani A (2008) Distinct roles of group I mGlu receptors in striatal function. Neuropharmacology 55(4):392–395. doi:10.1016/j.neuropharm.2008.05.020
Ribeiro FM, Paquet M, Cregan SP, Ferguson SS (2010) Group I metabotropic glutamate receptor signalling and its implication in neurological disease. CNS Neurol Disord Drug Targets 9(5):574–595
Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R (2004) Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci 24(13):3370–3378. doi:10.1523/JNEUROSCI.1633-03.2004
Pierozan P, Ferreira F, de Lima BO, Pessoa-Pureur R (2015) Quinolinic acid induces disrupts cytoskeletal homeostasis in striatal neurons. Protective role of astrocyte-neuron interaction. J Neurosci Res 93(2):268–284. doi:10.1002/jnr.23494
Huber F, Montani M, Sulser T, Jaggi R, Wild P, Moch H, Gevensleben H, Schmid M et al (2015) Comprehensive validation of published immunohistochemical prognostic biomarkers of prostate cancer —what has gone wrong? A blueprint for the way forward in biomarker studies. Br J Cancer 112(1):140–148. doi:10.1038/bjc.2014.588
Chang L, Goldman RD (2004) Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol 5(8):601–613. doi:10.1038/nrm1438
Tan L, Yu JT (2012) The kynurenine pathway in neurodegenerative diseases: mechanistic and therapeutic considerations. J Neurol Sci 323(1–2):1–8. doi:10.1016/j.jns.2012.08.005
Pierozan P, Fernandes CG, Dutra MF, Pandolfo P, Ferreira F, de Lima BO, Porciúncula L, Wajner M et al (2014) Biochemical, histopathological and behavioral alterations caused by intrastriatal administration of quinolic acid to young rats. FEBS J 281(5)
Pierozan P, Biasibetti H, Schmitz F, Avila H, Parisi MM, Barbe-Tuana F, Wyse AT, Pessoa-Pureur R (2016) Quinolinic acid neurotoxicity: differential roles of astrocytes and microglia via FGF-2-mediated signaling in redox-linked cytoskeletal changes. Biochim Biophys Acta 1863(12):3001–3014. doi:10.1016/j.bbamcr.2016.09.014
Pierozan P, Ferreira F, Ortiz de Lima B, Goncalves Fernandes C, Totarelli Monteforte P, de Castro MN, Bincoletto C, Soubhi Smaili S et al (2014) The phosphorylation status and cytoskeletal remodeling of striatal astrocytes treated with quinolinic acid. Exp Cell Res 322(2):313–323. doi:10.1016/j.yexcr.2014.02.024
Freese A, DiFiglia M, Koroshetz WJ, Beal MF, Martin JB (1990) Characterization and mechanism of glutamate neurotoxicity in primary striatal cultures. Brain Res 521(1–2):254–264
Lamprecht R (2016) The role of actin cytoskeleton in memory formation in amygdala. Front Mol Neurosci 9:23. doi:10.3389/fnmol.2016.00023
Chazeau A, Giannone G (2016) Organization and dynamics of the actin cytoskeleton during dendritic spine morphological remodeling. Cell Mol Life Sci 73(16):3053–3073. doi:10.1007/s00018-016-2214-1
Yuan A, Rao MV, Veeranna NRA (2017) Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol 9(4). doi:10.1101/cshperspect.a018309
Asahara H, Taniwaki T, Ohyagi Y, Yamada T, Kira J (1999) Glutamate enhances phosphorylation of neurofilaments in cerebellar granule cell culture. J Neurol Sci 171(2):84–87
Ackerley S, Grierson AJ, Brownlees J, Thornhill P, Anderton BH, Leigh PN, Shaw CE, Miller CC (2000) Glutamate slows axonal transport of neurofilaments in transfected neurons. J Cell Biol 150(1):165–176
Yano S, Fukunaga K, Ushio Y, Miyamoto E (1994) Activation of Ca2+/calmodulin-dependent protein kinase II and phosphorylation of intermediate filament proteins by stimulation of glutamate receptors in cultured rat cortical astrocytes. J Biol Chem 269(7):5428–5439
Kommers T, Rodnight R, Boeck C, Vendite D, Oliveira D, Horn J, Oppelt D, Wofchuk S (2002) Phosphorylation of glial fibrillary acidic protein is stimulated by glutamate via NMDA receptors in cortical microslices and in mixed neuronal/glial cell cultures prepared from the cerebellum. Developmental Brain Research 137(2):139–148
Chew SS, Johnson CS, Green CR, Danesh-Meyer HV (2010) Role of connexin43 in central nervous system injury. Exp Neurol 225(2):250–261. doi:10.1016/j.expneurol.2010.07.014
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by grants of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [grant number 303913/2013–4] and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) [grant number 11/0897–0].
Rights and permissions
About this article
Cite this article
Pierozan, P., Pessoa-Pureur, R. Cytoskeleton as a Target of Quinolinic Acid Neurotoxicity: Insight from Animal Models. Mol Neurobiol 55, 4362–4372 (2018). https://doi.org/10.1007/s12035-017-0654-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0654-8