Abstract
In the present study, we demonstrate that molecular chaperone Hsp70 and Hsc70 is essential for normal organization and development of ommatidial cells in Drosophila melanogaster eye. An exogenously expressed dominant negative mutant of Hsp70 (K71E) and Hsc70.4 (K71S and D206S) in an eye-specific manner resulted in eye degeneration that includes loss of eye pigment, disorganized ommatidia, abnormality in bristle cell arrangement and reduction in the eye size. The developmental organization of ommatidial cells (cone, photoreceptor, pigment, and bristle cell complex) was disturbed in Hsp70 and Hsc70 mutants. Acridine orange (AO) and caspase 3 staining showed an increased cell death in Hsp70 and Hsc70 mutant eyes. Genetic interaction study of Hsp70 and Hsc70 mutants with candidate genes of JNK signaling pathway and immunocytochemistry study using phospho-JNK antibody suggested that mutation in Hsp70 and Hsc70 results in ectopic activation of JNK signaling in fly eye. Further, anti-PH3 staining in Hsp70 and Hsc70 mutant eyes revealed a reduced number of mitotic cells in second mitotic wave (SMW) of developing eye and anti-Rh1 staining showed reduced Rh1 expression, accumulation of Rh1 in the cytoplasm, and rhabdomere degeneration. Thus, on the basis of results, it was concluded that molecular chaperone Hsp70 and Hsc70 play an indispensable role during Drosophila eye development.













Similar content being viewed by others
References
Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU (2013) Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem 82:323–355. doi:10.1146/annurev-biochem-060208-092442
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475(7356):324–332. doi:10.1038/nature10317
Chirico WJ, Waters MG, Blobel G (1988) 70K heat shock related proteins stimulate protein translocation into microsomes. Nature 332(6167):805–810. doi:10.1038/332805a0
Deshaies RJ, Koch BD, Werner-Washburne M, Craig EA, Schekman R (1988) A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature 332(6167):800–805. doi:10.1038/332800a0
Walton PA, Wendland M, Subramani S, Rachubinski RA, Welch WJ (1994) Involvement of 70-kD heat-shock proteins in peroxisomal import. J Cell Biol 125(5):1037–1046
Hunt C, Morimoto RI (1985) Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A 82(19):6455–6459
Rubin DM, Mehta AD, Zhu J, Shoham S, Chen X, Wells QR, Palter KB (1993) Genomic structure and sequence analysis of Drosophila melanogaster HSC70 genes. Gene 128(2):155–163
Shopland LS, Lis JT (1996) HSF recruitment and loss at most Drosophila heat shock loci is coordinated and depends on proximal promoter sequences. Chromosoma 105(3):158–171
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282. doi:10.1146/annurev.physiol.61.1.243
Lindquist S (1986) The heat-shock response. Annu Rev Biochem 55:1151–1191. doi:10.1146/annurev.bi.55.070186.005443
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677. doi:10.1146/annurev.ge.22.120188.003215
Shukla AK, Pragya P, Chaouhan HS, Tiwari AK, Patel DK, Abdin MZ, Chowdhuri DK Heat shock protein-70 (Hsp-70) suppresses paraquat-induced neurodegeneration by inhibiting JNK and caspase-3 activation in Drosophila model of Parkinson's disease. PLoS One 9(6):e98886. doi:10.1371/journal.pone.0098886
Gong WJ, Golic KG (2006) Loss of Hsp70 in Drosophila is pleiotropic, with effects on thermotolerance, recovery from heat shock and neurodegeneration. Genetics 172(1):275–286. doi:10.1534/genetics.105.048793
Cobreros L, Fernandez-Minan A, Luque CM, Gonzalez-Reyes A, Martin-Bermudo MD (2008) A role for the chaperone Hsp70 in the regulation of border cell migration in the Drosophila ovary. Mech Dev 125(11–12):1048–1058. doi:10.1016/j.mod.2008.07.006
Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM (2002) Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science 295(5556):865–868. doi:10.1126/science.1067389
Shukla AK, Pragya P, Chaouhan HS, Tiwari AK, Patel DK, Abdin MZ, Chowdhuri DK (2014) Heat shock protein-70 (Hsp-70) suppresses paraquat-induced neurodegeneration by inhibiting JNK and caspase-3 activation in Drosophila model of Parkinson’s disease. PLoS One 9(6):e98886. doi:10.1371/journal.pone.0098886
Zhang CW, Adeline HB, Chai BH, Hong ET, Ng CH, Lim KL (2016) Pharmacological or genetic activation of Hsp70 protects against loss of Parkin function. Neurodegener Dis 16(5–6):304–316. doi:10.1159/000443668
Elefant F, Palter KB (1999) Tissue-specific expression of dominant negative mutant Drosophila HSC70 causes developmental defects and lethality. Mol Biol Cell 10(7):2101–2117
Cremona O, De Camilli P (1997) Synaptic vesicle endocytosis. Curr Opin Neurobiol 7(3):323–330
Newmyer SL, Schmid SL (2001) Dominant-interfering Hsc70 mutants disrupt multiple stages of the clathrin-coated vesicle cycle in vivo. J Cell Biol 152(3):607–620
Rothnie A, Clarke AR, Kuzmic P, Cameron A, Smith CJ (2011) A sequential mechanism for clathrin cage disassembly by 70-kDa heat-shock cognate protein (Hsc70) and auxilin. Proc Natl Acad Sci U S A 108(17):6927–6932. doi:10.1073/pnas.1018845108
Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M (1998) Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J 17(21):6124–6134. doi:10.1093/emboj/17.21.6124
Belay HT, Brown IR (2006) Cell death and expression of heat-shock protein Hsc70 in the hyperthermic rat brain. J Neurochem 97(Suppl 1):116–119. doi:10.1111/j.1471-4159.2005.03591.x
Gain P, Thuret G, Chiquet C, Dumollard JM, Mosnier JF, Campos L (2001) In situ immunohistochemical study of Bcl-2 and heat shock proteins in human corneal endothelial cells during corneal storage. Br J Ophthalmol 85(8):996–1000
Bagchi M, Katar M, Maisel H (2002) Heat shock proteins of adult and embryonic human ocular lenses. J Cell Biochem 84(2):278–284
Kim JH, Yu YS, Chung H, Heo JW, Seo JS (2003) Effect of the absence of heat shock protein 70.1 (hsp70.1) on retinal photic injury. Korean J Ophthalmol 17(1):7–13. doi:10.3341/kjo.2003.17.1.7
Evans TG, Yamamoto Y, Jeffery WR, Krone PH (2005) Zebrafish Hsp70 is required for embryonic lens formation. Cell Stress Chaperones 10(1):66–78
Blechinger SR, Evans TG, Tang PT, Kuwada JY, Warren JT Jr, Krone PH (2002) The heat-inducible zebrafish hsp70 gene is expressed during normal lens development under non-stress conditions. Mech Dev 112(1–2):213–215
Kojima M, Hoshimaru M, Aoki T, Takahashi JB, Ohtsuka T, Asahi M, Matsuura N, Kikuchi H (1996) Expression of heat shock proteins in the developing rat retina. Neurosci Lett 205(3):215–217
Bernstein SL, Liu AM, Hansen BC, Somiari RI (2000) Heat shock cognate-70 gene expression declines during normal aging of the primate retina. Invest Ophthalmol Vis Sci 41(10):2857–2862
Gorbatyuk MS, Knox T, LaVail MM, Gorbatyuk OS, Noorwez SM, Hauswirth WW, Lin JH, Muzyczka N, Lewin AS (2010) Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proc Natl Acad Sci U S A 107(13):5961–5966. doi:10.1073/pnas.0911991107
Athanasiou D, Kosmaoglou M, Kanuga N, Novoselov SS, Paton AW, Paton JC, Chapple JP, Cheetham ME (2012) BiP prevents rod opsin aggregation. Mol Biol Cell 23(18):3522–3531. doi:10.1091/mbc.E12-02-0168
Ryoo HD, Domingos PM, Kang MJ, Steller H (2007) Unfolded protein response in a Drosophila model for retinal degeneration. EMBO J 26(1):242–252. doi:10.1038/sj.emboj.7601477
Hagedorn EJ, Bayraktar JL, Kandachar VR, Bai T, Englert DM, Chang HC (2006) Drosophila melanogaster auxilin regulates the internalization of delta to control activity of the Notch signaling pathway. J Cell Biol 173(3):443–452. doi:10.1083/jcb.200602054
Chang HC, Newmyer SL, Hull MJ, Ebersold M, Schmid SL, Mellman I (2002) Hsc70 is required for endocytosis and clathrin function in Drosophila. J Cell Biol 159(3):477–487. doi:10.1083/jcb.200205086
Cagan RL, Ready DF (1989) Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev 3(8):1099–1112
Cagan R (2009) Principles of Drosophila eye differentiation. Curr Top Dev Biol 89:115–135. doi:10.1016/S0070-2153(09)89005-4
Kumar JP (2012) Building an ommatidium one cell at a time. Dev Dyn 241(1):136–149. doi:10.1002/dvdy.23707
Gehring W (1966) Cell heredity and changes of determination in cultures of imaginal discs in Drosophila melanogaster. J Embryol Exp Morphol 15(1):77–111
Ouweneel WJ (1970) Normal and abnormal determination in the imaginal discs of Drosophila, with special reference to the eye discs. Acta Embryol Exp (Palermo) 1:95–119
Ready DF, Hanson TE, Benzer S (1976) Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol 53(2):217–240
Tomlinson A (1988) Cellular interactions in the developing Drosophila eye. Development 104(2):183–193
Roignant JY, Treisman JE (2009) Pattern formation in the Drosophila eye disc. Int J Dev Biol 53(5–6):795–804. doi:10.1387/ijdb.072483jr
Tomlinson A, Ready DF (1987) Cell fate in the Drosophila ommatidium. Dev Biol 123(1):264–275
Cagan RL, Ready DF (1989) The emergence of order in the Drosophila pupal retina. Dev Biol 136(2):346–362
Wolff T, Ready DF (1991) The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development 113(3):841–850
Wolff T, Ready DF (1991) Cell death in normal and rough eye mutants of Drosophila. Development 113(3):825–839
Miller DT, Cagan RL (1998) Local induction of patterning and programmed cell death in the developing Drosophila retina. Development 125(12):2327–2335
Larson DE, Johnson RI, Swat M, Cordero JB, Glazier JA, Cagan RL (2010) Computer simulation of cellular patterning within the Drosophila pupal eye. PLoS Comput Biol 6:e1000841. doi:10.1371/journal.pcbi.1000841
Freeman M (1996) Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 87(4):651–660
Song Z, Guan B, Bergman A, Nicholson DW, Thornberry NA, Peterson EP, Steller H (2000) Biochemical and genetic interactions between Drosophila caspases and the proapoptotic genes rpr, hid, and grim. Mol Cell Biol 20(8):2907–2914
Li LB, Xu K, Bonini NM (2007) Suppression of polyglutamine toxicity by the yeast Sup35 prion domain in Drosophila. J Biol Chem 282(52):37694–37701. doi:10.1074/jbc.M705211200
Warrick JM, Paulson HL, Gray-Board GL, Bui QT, Fischbeck KH, Pittman RN, Bonini NM (1998) Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell 93(6):939–949
Wang SL, Hawkins CJ, Yoo SJ, Muller HA, Hay BA (1999) The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell 98(4):453–463
Hay BA, Wolff T, Rubin GM (1994) Expression of baculovirus P35 prevents cell death in Drosophila. Development 120(8):2121–2129
Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martinez-Arias A (1998) Puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev 12(4):557–570
Adachi-Yamada T, Fujimura-Kamada K, Nishida Y, Matsumoto K (1999) Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature 400(6740):166–169. doi:10.1038/22112
Arya R, Lakhotia SC (2006) A simple nail polish imprint technique for examination of external morphology of Drosophila eyes. Curr Sci 90(9):1179–1180
Lakhotia SC, Prasanth KV (2002) Tissue- and development-specific induction and turnover of hsp70 transcripts from loci 87A and 87C after heat shock and during recovery in Drosophila melanogaster. J Exp Biol 205(Pt 3):345–358
Warrick JM, Morabito LM, Bilen J, Gordesky-Gold B, Faust LZ, Paulson HL, Bonini NM (2005) Ataxin-3 suppresses polyglutamine neurodegeneration in Drosophila by a ubiquitin-associated mechanism. Mol Cell 18(1):37–48. doi:10.1016/j.molcel.2005.02.030
Ray M, Lakhotia SC (2015) The commonly used eye-specific sev-GAL4 and GMR-GAL4 drivers in Drosophila melanogaster are expressed in tissues other than eyes also. J Genet 94(3):407–416
Li WZ, Li SL, Zheng HY, Zhang SP, Xue L (2012) A broad expression profile of the GMR-GAL4 driver in Drosophila melanogaster. Genet Mol Res 11(3):1997–2002. doi:10.4238/2012.August.6.4
Arikawa K, Hicks JL, Williams DS (1990) Identification of actin filaments in the rhabdomeral microvilli of Drosophila photoreceptors. J Cell Biol 110(6):1993–1998
Kumar JP, Ready DF (1995) Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development 121(12):4359–4370
Bahri SM, Yang X, Chia W (1997) The Drosophila bifocal gene encodes a novel protein which colocalizes with actin and is necessary for photoreceptor morphogenesis. Mol Cell Biol 17(9):5521–5529
Xiong B, Bellen HJ (2013) Rhodopsin homeostasis and retinal degeneration: lessons from the fly. Trends Neurosci 36(11):652–660. doi:10.1016/j.tins.2013.08.003
Komarova EY, Afanasyeva EA, Bulatova MM, Cheetham ME, Margulis BA, Guzhova IV (2004) Downstream caspases are novel targets for the antiapoptotic activity of the molecular chaperone hsp70. Cell Stress Chaperones 9(3):265–275
Hohfeld J (1998) Regulation of the heat shock conjugate Hsc70 in the mammalian cell: the characterization of the anti-apoptotic protein BAG-1 provides novel insights. Biol Chem 379(3):269–274
Arya R, Mallik M, Lakhotia SC (2007) Heat shock genes—integrating cell survival and death. J Biosci 32(3):595–610
Grzeschik NA, Knust E (2005) IrreC/rst-mediated cell sorting during Drosophila pupal eye development depends on proper localisation of DE-cadherin. Development 132(9):2035–2045. doi:10.1242/dev.01800
Larson DE, Liberman Z, Cagan RL (2008) Cellular behavior in the developing Drosophila pupal retina. Mech Dev 125(3–4):223–232. doi:10.1016/j.mod.2007.11.007
Tepass U, Harris KP (2007) Adherens junctions in Drosophila retinal morphogenesis. Trends Cell Biol 17(1):26–35. doi:10.1016/j.tcb.2006.11.006
Myers KR, Casanova JE (2008) Regulation of actin cytoskeleton dynamics by Arf-family GTPases. Trends Cell Biol 18(4):184–192. doi:10.1016/j.tcb.2008.02.002
Riggs B, Rothwell W, Mische S, Hickson GR, Matheson J, Hays TS, Gould GW, Sullivan W (2003) Actin cytoskeleton remodeling during early Drosophila furrow formation requires recycling endosomal components nuclear-fallout and Rab11. J Cell Biol 163(1):143–154. doi:10.1083/jcb.200305115
Xiang W, Rensing L (1999) Changes in cell morphology and actin organization during heat shock in Dictyostelium discoideum: does HSP70 play a role in acquired thermotolerance? FEMS Microbiol Lett 178(1):95–107
Warrick JM, Chan HY, Gray-Board GL, Chai Y, Paulson HL, Bonini NM (1999) Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat Genet 23(4):425–428. doi:10.1038/70532
Mitra A, Chinchore Y, Kinser R, Dolph PJ Characterization of two dominant alleles of the major rhodopsin-encoding gene ninaE in Drosophila. Mol Vis 17:3224–3233
Chang HY, Ready DF (2000) Rescue of photoreceptor degeneration in rhodopsin-null Drosophila mutants by activated Rac1. Science 290(5498):1978–1980
Acknowledgments
We thank Prof. J. K. Roy (Banaras Hindu University, Varanasi, India), Prof. Nancy M. Bonini (Howard Hughes Medical Institute) for UAS-Hsp70 K71E, Prof. Drick Bohman (University of Rochester Medical Center, Rochester, NY USA) for UAS-Bsk DN, Prof. Martinez-Arias (University of Cambridge, UK) for puc E69, Prof. M. Miura (Graduate Institute of Pharmaceuticals Sciences, University of Tokyo) for UAS-eiger, and Bloomington Drosophila stock Centre (Indiana University, 1001 E. Third St. Bloomington, USA) for fly stocks. We also thank Prof. Patric Dolph (Dart Morth College Medical School, USA) for anti-Rh1 antibody. The financial assistance from Department of Science & Technology (DST), New Delhi, India, to AKT (SR/FT/LS-1/2010), Laser Scanning Confocal Microscope facility supported by the Department of Biotechnology (DBT), India, and support from The Puri Foundation for Education in India is duly acknowledged.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: AK and AKT. Performed the experiments: AK. Analyzed the data: AK and AKT. Wrote the paper: AK and AKT. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Funding
This work was supported by the Department of Science and Technology (DST), New Delhi, India, to AKT (SR/FT/LS-1/2010).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Electronic Supplementary Material
Supplementary Figure 1
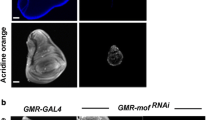
Mutation in Hsp70 and Hsc70 results in excessive cell death in larval and pupal eyes. (a-c) AO staining in third instar larval eye imaginal disc of Oregon R+ (a), UAS-Hsp70 K71E -GMR-GAL4/+ (b) and UAS-Hsc70.4 K71S -GMR-GAL4/+ (c). (d-f) AO staining in 50 hr pupal eyes from Oregon R+ (d), UAS-Hsp70 K71E -GMR-GAL4/+ (e) and UAS-Hsc70.4 K71S -GMR-GAL4/+ (f). Eyes from Hsp70 and Hsc70 mutants showed an increase cell death. AO staining seen at the periphery of pupal eyes in contol represents the developmental apoptosis (d, arrow). Scale Bar represents 100 μm (a-f). (Total 10 eyes (larval & pupal) were used for cell death observations). (TIFF 4717 kb)
Supplementary Figure 2
F-actin (Green) and anti-Hsp70 (5A5) (Red) staining in 50 h pupal eyes of control, Hsp70 and Hsc70 mutants. (a-d) F-actin stained 50 h pupal eyes of GMR-GAL4/+ showing proper organization of cone, pigment and bristle cell (a), a severely disorganized cone, pigment and bristle cells with actin accumulation in UAS-Hsp70 K71E -GMR-GAL4/+ eyes (b, arrow), a less severe disorganized cone, pigment and bristle cells with less actin accumulation in UAS-Hsc70.4 K71S -GMR-GAL4/+ eye was observed (c, arrow) while in GMR-GAL4/+;UAS-Hsc70.4 RNAi /+ less affected actin cytoskeleton and normally arranged cone, pigment and bristle cells were observed (d). (e-h) Anti-Hsp70 staining in GMR-GAL4/+ showing basal level of Hsp70 protein expression in GMR-GAL4/+ (e), depleted Hsp70 protein in UAS-Hsp70 K71E -GMR-GAL4/+ (f, arrow), slightly upregulated Hsp70 protein in UAS-Hsc70.4 K71S -GMR-GAL4/+ (g) and strongly upregulated Hsp70 protein in GMR-GAL4/+;UAS-Hsc70.4 RNAi /+eye (h). (i-l) are the merged images of (a & e), (b & f), (c & g) and (d & h) respectively. Scale bars represent 20 μm (a-l). (Total 10 pupal eyes were used from each group). (TIFF 4539 kb)
Supplementary Table 1
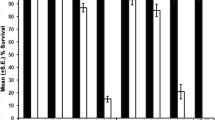
Various phenotype shown by Hsc70 (Hsc70.4) and Hsp70 mutants driven by GMR-GAL4. (DOC 37 kb)
Rights and permissions
About this article
Cite this article
Kumar, A., Tiwari, A.K. Molecular Chaperone Hsp70 and Its Constitutively Active Form Hsc70 Play an Indispensable Role During Eye Development of Drosophila melanogaster . Mol Neurobiol 55, 4345–4361 (2018). https://doi.org/10.1007/s12035-017-0650-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0650-z