Abstract
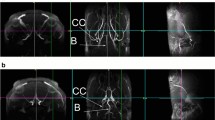
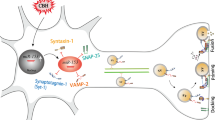
Chronic cerebral hypoperfusion (CCH) evokes mild cognitive impairment (MCI) and contributes to the progression of vascular dementia and Alzheimer’s disease (AD). How CCH induces these neurodegenerative processes that may spread along the synaptic network and whether they are detectable at the synaptic proteome level of the cerebral cortex remains to be established. In the present study, we report the synaptic protein changes in the cerebral cortex after stepwise bilateral common carotid artery occlusion (BCCAO) induced CCH in the rat. The occlusions were confirmed with magnetic resonance angiography 5 weeks after the surgery. Synaptosome fractions were prepared using sucrose gradient centrifugation from cerebral cortex dissected 7 weeks after the occlusion. The synaptic protein differences between the sham operated and CCH groups were analyzed with label-free nanoUHPLC-MS/MS. We identified 46 proteins showing altered abundance due to CCH. In particular, synaptic protein and lipid metabolism, as well as GABA shunt-related proteins showed increased while neurotransmission and synaptic assembly-related proteins showed decreased protein level changes in CCH rats. Protein network analysis of CCH-induced protein alterations suggested the importance of increased synaptic apolipoprotein E (APOE) level as a consequence of CCH. Therefore, the change in APOE level was confirmed with Western blotting. The identified synaptic protein changes would precede the onset of dementia-like symptoms in the CCH model, suggesting their importance in the development of vascular dementia.





Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- APOE:
-
Apolipoprotein E
- BCCAO:
-
Bilateral common carotid artery occlusion
- CCH:
-
Chronic cerebral hypoperfusion
References
Cankurtaran M, Yavuz BB, Cankurtaran ES, Halil M, Ulger Z, Ariogul S (2008) Risk factors and type of dementia: vascular or Alzheimer? Arch Gerontol Geriatr 47:25–34
Valerio Romanini C, Dias Fiuza Ferreira E, Correia Bacarin C, Verussa MH, Weffort de Oliveira RM, Milani H (2013) Neurohistological and behavioral changes following the four-vessel occlusion/internal carotid artery model of chronic cerebral hypoperfusion: comparison between normotensive and spontaneously hypertensive rats. Behav Brain Res 252:214–221
Sato N, Morishita R (2013) Roles of vascular and metabolic components in cognitive dysfunction of Alzheimer disease: short- and long-term modification by non-genetic risk factors. Front Aging Neurosci 5:64
Borroni B, Anchisi D, Paghera B, Vicini B, Kerrouche N, Garibotto V, Terzi A, Vignolo LA et al (2006) Combined 99mTc-ECD SPECT and neuropsychological studies in MCI for the assessment of conversion to AD. Neurobiol Aging 27:24–31
Zadori D, Datki Z, Penke B (2007) The role of chronic brain hypoperfusion in the pathogenesis of Alzheimer’s disease—facts and hypotheses. Ideggyogy Sz 60:428–437
Shankar GM, Walsh DM (2009) Alzheimer's disease: synaptic dysfunction and Abeta. Mol Neurodegener 4:48
Farkas E, Luiten PG, Bari F (2007) Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 54:162–180
Jing Z, Shi C, Zhu L, Xiang Y, Chen P, Xiong Z, Li W, Ruan Y et al (2015) Chronic cerebral hypoperfusion induces vascular plasticity and hemodynamics but also neuronal degeneration and cognitive impairment. J Cereb Blood Flow Metab 35:1249–1259
Cechetti F, Worm PV, Pereira LO, Siqueira IR, Netto AC (2010) The modified 2VO ischemia protocol causes cognitive impairment similar to that induced by the standard method, but with a better survival rate. Braz J Med Biol Res 43:1178–1183
de la Torre JC, Pappas BA, Prevot V, Emmerling MR, Mantione K, Fortin T, Watson MD, Stefano GB (2003) Hippocampal nitric oxide upregulation precedes memory loss and A beta 1-40 accumulation after chronic brain hypoperfusion in rats. Neurol Res 25:635–641
Wang X, Lin F, Gao Y, Lei H (2015) Bilateral common carotid artery occlusion induced brain lesions in rats: a longitudinal diffusion tensor imaging study. Magn Reson Imaging 33:551–558
Okamoto Y, Yamamoto T, Kalaria RN, Senzaki H, Maki T, Hase Y, Kitamura A, Washida K et al (2012) Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol 123:381–394
Zhiyou C, Yong Y, Shanquan S, Jun Z, Liangguo H, Ling Y, Jieying L (2009) Upregulation of BACE1 and beta-amyloid protein mediated by chronic cerebral hypoperfusion contributes to cognitive impairment and pathogenesis of Alzheimer’s disease. Neurochem Res 34:1226–1235
Hahn CG, Banerjee A, Macdonald ML, Cho DS, Kamins J, Nie Z, Borgmann-Winter KE, Grosser T et al (2009) The post-synaptic density of human postmortem brain tissues: an experimental study paradigm for neuropsychiatric illnesses. PLoS One 4:e5251
Volgyi K, Gulyassy P, Haden K, Kis V, Badics K, Kekesi KA, Simor A, Gyorffy B et al (2015) Synaptic mitochondria: a brain mitochondria cluster with a specific proteome. J Proteome 120:142–157
Wisniewski JR, Zougman A, Nagaraj N, Mann M (2009) Universal sample preparation method for proteome analysis. Nat Methods 6:359–362
Soria G, Tudela R, Marquez-Martin A, Camon L, Batalle D, Munoz-Moreno E, Eixarch E, Puig J et al (2013) The ins and outs of the BCCAo model for chronic hypoperfusion: a multimodal and longitudinal MRI approach. PLoS One 8:e74631
Roberts RC, Roche JK, McCullumsmith RE (2014) Localization of excitatory amino acid transporters EAAT1 and EAAT2 in human postmortem cortex: a light and electron microscopic study. Neuroscience 277:522–540
Scimemi A (2014) Structure, function, and plasticity of GABA transporters. Front Cell Neurosci 8:161
Misgeld U, Bijak M, Jarolimek W (1995) A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol 46:423–462
Kulik A, Vida I, Lujan R, Haas CA, Lopez-Bendito G, Shigemoto R, Frotscher M (2003) Subcellular localization of metabotropic GABA(B) receptor subunits GABA(B1a/b) and GABA(B2) in the rat hippocampus. J Neurosci 23:11026–11035
Lu Y, Li CJ, Chen C, Luo P, Zhou M, Li C, Xu XL, Lu Q et al (2016) Activation of GABAB2 subunits alleviates chronic cerebral hypoperfusion-induced anxiety-like behaviours: A role for BDNF signalling and Kir3 channels. Neuropharmacology 110:308–321
Luo P, Chen C, Lu Y, Fu T, Lu Q, Xu X, Li C, He Z et al (2016) Baclofen ameliorates spatial working memory impairments induced by chronic cerebral hypoperfusion via up-regulation of HCN2 expression in the PFC in rats. Behav Brain Res 308:6–13
Liu L, Li CJ, Lu Y, Zong XG, Luo C, Sun J, Guo LJ (2015) Baclofen mediates neuroprotection on hippocampal CA1 pyramidal cells through the regulation of autophagy under chronic cerebral hypoperfusion. Sci Rep 5:14474
Li CJ, Lu Y, Zhou M, Zong XG, Li C, Xu XL, Guo LJ, Lu Q (2014) Activation of GABAB receptors ameliorates cognitive impairment via restoring the balance of HCN1/HCN2 surface expression in the hippocampal CA1 area in rats with chronic cerebral hypoperfusion. Mol Neurobiol 50:704–720
Bahn JH, Kwon OS, Joo HM, Ho Jang S, Park J, Hwang IK, Kang TC, Won MH et al (2002) Immunohistochemical studies of brain pyridoxine-5′-phosphate oxidase. Brain Res 925:159–168
Sweatt AJ, Garcia-Espinosa MA, Wallin R, Hutson SM (2004) Branched-chain amino acids and neurotransmitter metabolism: expression of cytosolic branched-chain aminotransferase (BCATc) in the cerebellum and hippocampus. J Comp Neurol 477:360–370
Picklo MJ Sr, Olson SJ, Hayes JD, Markesbery WR, Montine TJ (2001) Elevation of AKR7A2 (succinic semialdehyde reductase) in neurodegenerative disease. Brain Res 916:229–238
Huang Y, Mucke L (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148:1204–1222
Linnertz C, Anderson L, Gottschalk W, Crenshaw D, Lutz MW, Allen J, Saith S, Mihovilovic M et al (2014) The cis-regulatory effect of an Alzheimer’s disease-associated poly-T locus on expression of TOMM40 and apolipoprotein E genes. Alzheimers Dement 10:541–551
Laske C (2012) Clinical and biomarker changes in Alzheimer’s disease. N Engl J Med 367:2050 author reply 2051-2052
Martinez-Morillo E, Hansson O, Atagi Y, Bu G, Minthon L, Diamandis EP, Nielsen HM (2014) Total apolipoprotein E levels and specific isoform composition in cerebrospinal fluid and plasma from Alzheimer’s disease patients and controls. Acta Neuropathol 127:633–643
Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E et al (2012) Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149:708–721
Chen Y, Durakoglugil MS, Xian X, Herz J (2010) ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci U S A 107:12011–12016
Roses AD, Saunders AM, Lutz MW, Zhang N, Hariri AR, Asin KE, Crenshaw DG, Budur K et al (2014) New applications of disease genetics and pharmacogenetics to drug development. Curr Opin Pharmacol 14:81–89
Maruszak A, Peplonska B, Safranow K, Chodakowska-Zebrowska M, Barcikowska M, Zekanowski C (2012) TOMM40 rs10524523 polymorphism’s role in late-onset Alzheimer’s disease and in longevity. J Alzheimers Dis 28:309–322
Dhillon VS, Fenech M (2014) Mutations that affect mitochondrial functions and their association with neurodegenerative diseases. Mutat Res Rev Mutat Res 759:1–13
Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W Jr, Kaye J, Manczak M (2005) Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis 7:103–117 discussion 173-180
Xu PT, Li YJ, Qin XJ, Kroner C, Green-Odlum A, Xu H, Wang TY, Schmechel DE et al (2007) A SAGE study of apolipoprotein E3/3, E3/4 and E4/4 allele-specific gene expression in hippocampus in Alzheimer disease. Mol Cell Neurosci 36:313–331
Yamazaki M, Matsuo R, Fukazawa Y, Ozawa F, Inokuchi K (2001) Regulated expression of an actin-associated protein, synaptopodin, during long-term potentiation. J Neurochem 79:192–199
Connelly SJ, Mukaetova-Ladinska EB, Abdul-All Z, Alves da Silva J, Brayne C, Honer WG, Mann DM (2011) Synaptic changes in frontotemporal lobar degeneration: correlation with MAPT haplotype and APOE genotype. Neuropathol Appl Neurobiol 37:366–380
Furuya TK, Silva PN, Payao SL, Bertolucci PH, Rasmussen LT, De Labio RW, Braga IL, Chen ES et al (2012) Analysis of SNAP25 mRNA expression and promoter DNA methylation in brain areas of Alzheimer’s disease patients. Neuroscience 220:41–46
Guerini FR, Agliardi C, Sironi M, Arosio B, Calabrese E, Zanzottera M, Bolognesi E, Ricci C et al (2014) Possible association between SNAP-25 single nucleotide polymorphisms and alterations of categorical fluency and functional MRI parameters in Alzheimer’s disease. J Alzheimers Dis 42:1015–1028
Fan HP, Fan FJ, Bao L, Pei G (2006) SNAP-25/syntaxin 1A complex functionally modulates neurotransmitter gamma-aminobutyric acid reuptake. J Biol Chem 281:28174–28184
Chapman ER, An S, Barton N, Jahn R (1994) SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils. J Biol Chem 269:27427–27432
Tapiola T, Pennanen C, Tapiola M, Tervo S, Kivipelto M, Hanninen T, Pihlajamaki M, Laakso MP et al (2008) MRI of hippocampus and entorhinal cortex in mild cognitive impairment: a follow-up study. Neurobiol Aging 29:31–38
Skoog I, Hesse C, Fredman P, Andreasson LA, Palmertz B, Blennow K (1997) Apolipoprotein E in cerebrospinal fluid in 85-year-old subjects. Relation to dementia, apolipoprotein E polymorphism, cerebral atrophy, and white matter lesions. Arch Neurol 54:267–272
Kielian T, Esen N (2004) Effects of neuroinflammation on glia-glia gap junctional intercellular communication: a perspective. Neurochem Int 45:429–436
Iacobas DA, Iacobas S, Urban-Maldonado M, Spray DC (2005) Sensitivity of the brain transcriptome to connexin ablation. Biochim Biophys Acta 1711:183–196
Leithe E, Rivedal E (2004) Ubiquitination and down-regulation of gap junction protein connexin-43 in response to 12-O-tetradecanoylphorbol 13-acetate treatment. J Biol Chem 279:50089–50096
Machtaler S, Dang-Lawson M, Choi K, Jang C, Naus CC, Matsuuchi L (2011) The gap junction protein Cx43 regulates B-lymphocyte spreading and adhesion. J Cell Sci 124:2611–2621
Morioka N, Zhang FF, Nakamura Y, Kitamura T, Hisaoka-Nakashima K, Nakata Y (2015) Tumor necrosis factor-mediated downregulation of spinal astrocytic connexin43 leads to increased glutamatergic neurotransmission and neuropathic pain in mice. Brain Behav Immun 49:293–310
Boyd-Kimball D, Castegna A, Sultana R, Poon HF, Petroze R, Lynn BC, Klein JB, Butterfield DA (2005) Proteomic identification of proteins oxidized by Abeta(1-42) in synaptosomes: implications for Alzheimer’s disease. Brain Res 1044:206–215
Rose EM, Koo JC, Antflick JE, Ahmed SM, Angers S, Hampson DR (2009) Glutamate transporter coupling to Na,K-ATPase. J Neurosci 29:8143–8155
Mace PD, Wallez Y, Egger MF, Dobaczewska MK, Robinson H, Pasquale EB, Riedl SJ (2013) Structure of ERK2 bound to PEA-15 reveals a mechanism for rapid release of activated MAPK. Nat Commun 4:1681
Rohe M, Carlo AS, Breyhan H, Sporbert A, Militz D, Schmidt V, Wozny C, Harmeier A et al (2008) Sortilin-related receptor with A-type repeats (SORLA) affects the amyloid precursor protein-dependent stimulation of ERK signaling and adult neurogenesis. J Biol Chem 283:14826–14834
Ahn EH, Kim DW, Shin MJ, Kim HR, Kim SM, Woo SJ, Eom SA, Jo HS et al (2014) PEP-1-PEA-15 protects against toxin-induced neuronal damage in a mouse model of Parkinson’s disease. Biochim Biophys Acta 1840:1686–1700
Habelhah H, Shah K, Huang L, Ostareck-Lederer A, Burlingame AL, Shokat KM, Hentze MW, Ronai Z (2001) ERK phosphorylation drives cytoplasmic accumulation of hnRNP-K and inhibition of mRNA translation. Nat Cell Biol 3:325–330
Liang D, Han G, Feng X, Sun J, Duan Y, Lei H (2012) Concerted perturbation observed in a hub network in Alzheimer’s disease. PLoS One 7:e40498
Chen HC, Lin WC, Tsay YG, Lee SC, Chang CJ (2002) An RNA helicase, DDX1, interacting with poly(A) RNA and heterogeneous nuclear ribonucleoprotein K. J Biol Chem 277:40403–40409
Taniguchi M, Okayama Y, Hashimoto Y, Kitaura M, Jimbo D, Wakutani Y, Wada-Isoe K, Nakashima K et al (2008) Sugar chains of cerebrospinal fluid transferrin as a new biological marker of Alzheimer’s disease. Dement Geriatr Cogn Disord 26:117–122
Booyjzsen C, Scarff CA, Moreton B, Portman I, Scrivens JH, Costantini G, Sadler PJ (2012) Fibrillation of transferrin. Biochim Biophys Acta 1820:427–436
Khachaturian ZS (2008) Alzheimer’s & dementia: the Journal of the Alzheimer’s Association. Alzheimers Dement 4:315
Conner SD, Schmid SL (2002) Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J Cell Biol 156:921–929
Fernandez-Chacon R, Achiriloaie M, Janz R, Albanesi JP, Sudhof TC (2000) SCAMP1 function in endocytosis. J Biol Chem 275:12752–12756
Davidson JO, Green CR, Bennet L, Nicholson LF, Danesh-Meyer H, O'Carroll SJ, Gunn AJ (2013) A key role for connexin hemichannels in spreading ischemic brain injury. Curr Drug Targets 14:36–46
Masaki K, Suzuki SO, Matsushita T, Matsuoka T, Imamura S, Yamasaki R, Suzuki M, Suenaga T et al (2013) Connexin 43 astrocytopathy linked to rapidly progressive multiple sclerosis and neuromyelitis optica. PLoS One 8:e72919
Wallach G, Lallouette J, Herzog N, De Pitta M, Ben Jacob E, Berry H, Hanein Y (2014) Glutamate mediated astrocytic filtering of neuronal activity. PLoS Comput Biol 10:e1003964
Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA et al (1999) Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A 96:15233–15238
Ophir G, Meilin S, Efrati M, Chapman J, Karussis D, Roses A, Michaelson DM (2003) Human apoE3 but not apoE4 rescues impaired astrocyte activation in apoE null mice. Neurobiol Dis 12:56–64
Zekonyte J, Sakai K, Nicoll JA, Weller RO, Carare RO (2016) Quantification of molecular interactions between ApoE, amyloid-beta (Abeta) and laminin: relevance to accumulation of Abeta in Alzheimer’s disease. Biochim Biophys Acta 1862:1047–1053
Tai LM, Ghura S, Koster KP, Liakaite V, Maienschein-Cline M, Kanabar P, Collins N, Ben-Aissa M et al (2015) APOE-modulated Abeta-induced neuroinflammation in Alzheimer’s disease: current landscape, novel data, and future perspective. J Neurochem 133:465–488
Murphy MP, Corriveau RA, Wilcock DM (2016) Vascular contributions to cognitive impairment and dementia (VCID). Biochim Biophys Acta 1862:857–859
Chihara T, Luginbuhl D, Luo L (2007) Cytoplasmic and mitochondrial protein translation in axonal and dendritic terminal arborization. Nat Neurosci 10:828–837
Folci A, Mapelli L, Sassone J, Prestori F, D'Angelo E, Bassani S, Passafaro M (2014) Loss of hnRNP K impairs synaptic plasticity in hippocampal neurons. J Neurosci 34:9088–9095
Tanaka S, Uehara T, Nomura Y (2000) Up-regulation of protein-disulfide isomerase in response to hypoxia/brain ischemia and its protective effect against apoptotic cell death. J Biol Chem 275:10388–10393
Ding M, Shen K (2008) The role of the ubiquitin proteasome system in synapse remodeling and neurodegenerative diseases. BioEssays 30:1075–1083
Na CH, Jones DR, Yang Y, Wang X, Xu Y, Peng J (2012) Synaptic protein ubiquitination in rat brain revealed by antibody-based ubiquitome analysis. J Proteome Res 11:4722–4732
Lyon RC, Johnston SM, Watson DG, McGarvie G, Ellis EM (2007) Synthesis and catabolism of gamma-hydroxybutyrate in SH-SY5Y human neuroblastoma cells: role of the aldo-keto reductase AKR7A2. J Biol Chem 282:25986–25992
Manya H, Aoki J, Watanabe M, Adachi T, Asou H, Inoue Y, Arai H, Inoue K (1998) Switching of platelet-activating factor acetylhydrolase catalytic subunits in developing rat brain. J Biol Chem 273:18567–18572
Kamada H, Sato K, Zhang WR, Omori N, Nagano I, Shoji M, Abe K (2003) Spatiotemporal changes of apolipoprotein E immunoreactivity and apolipoprotein E mRNA expression after transient middle cerebral artery occlusion in rat brain. J Neurosci Res 73:545–556
McKenna MC, Stevenson JH, Huang XL, Tildon JT, Zielke CL, Hopkins IB (2000) Mitochondrial malic enzyme activity is much higher in mitochondria from cortical synaptic terminals compared with mitochondria from primary cultures of cortical neurons or cerebellar granule cells. Neurochem Int 36:451–459
Christel CJ, Schaer R, Wang S, Henzi T, Kreiner L, Grabs D, Schwaller B, Lee A (2012) Calretinin regulates Ca2+-dependent inactivation and facilitation of Ca(v)2.1 Ca2+ channels through a direct interaction with the alpha12.1 subunit. J Biol Chem 287:39766–39775
Acknowledgements
This study was supported by Gedeon Richter Plc, the Hungarian National Research, Development and Innovation Office (KMOP-1.1.5-08-2009-0001, KTIA_NAP_13-1-2013-0001, KTIA_NAP_B_13-2-2014-0004 and KTIA_NAP_13-2-2015-0003 programs). The founders had no role in the study design; in the collection, analysis and interpretation of data; in writing of the manuscript; and in the decision to submit the article for publication. We would like to thank Dr. Gábor Juhász for helpful discussions on the research plan.
Compliance with Ethical Guidelines
The care and experimentation of all animals conformed to the Hungarian Act of Animal Care and Experimentation (1998, XXVIII) and to the guidelines of the European Communities Council Directive, 86/609/EEC as well as with local regulations for the care and use of animals for research.
Author information
Authors and Affiliations
Corresponding author
Additional information
László Drahos and Arpád Dobolyi share senior author responsibilities.
Rights and permissions
About this article
Cite this article
Völgyi, K., Gulyássy, P., Todorov, M.I. et al. Chronic Cerebral Hypoperfusion Induced Synaptic Proteome Changes in the rat Cerebral Cortex. Mol Neurobiol 55, 4253–4266 (2018). https://doi.org/10.1007/s12035-017-0641-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0641-0