Abstract
Differentiation of pluripotent stem cells (PSCs) to neural lineages has gathered huge attention in both basic research and regenerative medicine. The major hurdle lies in the efficiency of differentiation and identification of small molecules that facilitate neurogenesis would partly circumvent this limitation. The small molecule Cyclosporine A (CsA), a commonly used immunosuppressive drug, has been shown to enhance in vivo neurogenesis. To extend the information to in vitro neurogenesis, we examined the effect of CsA on neural differentiation of PSCs. We found CsA to increase the expression of neural progenitor genes during early neural differentiation. Gene silencing approach revealed CsA-mediated neural induction to be dependent on blocking the Ca2+-activated phosphatase calcineurin (Cn) signaling. Similar observation with FK506, an independent inhibitor of Cn, further strengthened the necessity of blocking Cn for enhanced neurogenesis. Surprisingly, mechanistic insight revealed Cn-inhibition dependent upregulation of IL-6 protein to be necessary for CsA-mediated neurogenesis. Together, these findings provide a comprehensive understanding of the role of CsA in neurogenesis, thus suggesting a method for obtaining large numbers of neural progenitors from PSCs for possible transplantation.





Similar content being viewed by others
Abbreviations
- PSC:
-
Pluripotent stem cells
- iPSC:
-
Induced pluripotent stem cells
- miPSC:
-
Mouse induced pluripotent stem cells
- CsA:
-
Cyclosporine A
- Cn:
-
Calcineurin
- NFAT:
-
Nuclear factor of activated T cells
- ESC:
-
Embryonic stem cells
- iMEF:
-
Inactivated mouse embryonic fibroblasts
- LIF:
-
Leukemia inhibitory factor
- EB:
-
Embryoid body
References
Stewart R, Stojkovic M, Lako M (2006) Mechanisms of self-renewal in human embryonic stem cells. Eur J Cancer 42:1257–1272. doi:10.1016/j.ejca.2006.01.033
Avior Y, Sagi I, Benvenisty N (2016) Pluripotent stem cells in disease modelling and drug discovery. Nat Rev Mol Cell Biol 17:170–182. doi:10.1038/nrm.2015.27
Chuang J-H (2015) Neural differentiation from embryonic stem cells in vitro: an overview of the signaling pathways. World Journal of Stem Cells 7:437. doi:10.4252/wjsc.v7.i2.437
Elkabetz Y, Panagiotakos G, Al Shamy G et al (2008) Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev 22:152–165. doi:10.1101/gad.1616208
Engberg N, Kahn M, Petersen DR et al (2010) Retinoic acid synthesis promotes development of neural progenitors from mouse embryonic stem cells by suppressing endogenous, Wnt-dependent nodal signaling. Stem Cells 28:1498–1509. doi:10.1002/stem.479
Dhara SK, Stice SL (2008) Neural differentiation of human embryonic stem cells. J Cell Biochem 105:633–640. doi:10.1002/jcb.21891
Gorba T, Allsopp TE (2003) Pharmacological potential of embryonic stem cells. Pharmacology Research 47:269–278
Cezar GG (2007) Can human embryonic stem cells contribute to the discovery of safer and more effective drugs? Curr Opin Chem Biol 11:405–409. doi:10.1016/j.cbpa.2007.05.033
Chambers SM, Qi Y, Mica Y et al (2012) Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol 30:715–720. doi:10.1038/nbt.2249
Hu B-Y, Zhang S-C (2009) Differentiation of spinal motor neurons from pluripotent human stem cells. Nat Protoc 4:1295–1304. doi:10.1038/nprot.2009.127
Öström M, Loffler KA, Edfalk S et al (2008) Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into β-cells. PLoS One 3:e2841. doi:10.1371/journal.pone.0002841
Smith JR, Vallier L, Lupo G et al (2008) Inhibition of activin/nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol 313:107–117. doi:10.1016/j.ydbio.2007.10.003
Zhou J, Su P, Li D et al (2010) High-efficiency induction of neural conversion in human ESCs and human induced pluripotent stem cells with a single chemical inhibitor of transforming growth factor Beta superfamily receptors. Stem Cells 28:1741–1750. doi:10.1002/stem.504
Osakada F, Ikeda H, Mandai M et al (2008) Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol 26:215–224. doi:10.1038/nbt1384
Surmacz B, Fox H, Gutteridge A et al (2012) Directing differentiation of human embryonic stem cells toward anterior neural ectoderm using small molecules. Stem Cells 30:1875–1884. doi:10.1002/stem.1166
Li X, Zhu L, Yang A et al (2011) Calcineurin-NFAT signaling critically regulates early lineage specification in mouse embryonic stem cells and embryos. Cell Stem Cell 8:46–58. doi:10.1016/j.stem.2010.11.027
Sachinidis A, Schwengberg S, Hippler-Altenburg R et al (2006) Identification of small signalling molecules promoting cardiac-specific differentiation of mouse embryonic stem cells. Cell Physiol Biochem 18:303–314. doi:10.1159/000097608
Wang H (1999) Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 284:339–343. doi:10.1126/science.284.5412.339
Oh-hora M, Rao A (2009) The calcium/NFAT pathway: role in development and function of regulatory T cells. Microbes Infect 11:612–619. doi:10.1016/j.micinf.2009.04.008
Sachewsky N, Hunt J, Cooke MJ et al (2014) Cyclosporin A enhances neural precursor cell survival in mice through a calcineurin-independent pathway. Dis Model Mech 7:953–961. doi:10.1242/dmm.014480
Hunt J, Cheng A, Hoyles A et al (2010) Cyclosporin A has direct effects on adult neural precursor cells. J Neurosci 30:2888–2896. doi:10.1523/JNEUROSCI.5991-09.2010
Gómez-Sintes R, Lucas JJ (2010) NFAT/Fas signaling mediates the neuronal apoptosis and motor side effects of GSK-3 inhibition in a mouse model of lithium therapy. J Clin Investig 120:2432–2445. doi:10.1172/JCI37873
Declercq J, Sheshadri P, Verfaillie CM, Kumar A (2013) Zic3 enhances the generation of mouse induced pluripotent stem cells. Stem Cells Dev 22:2017–2025. doi:10.1089/scd.2012.0651
Lu J, Tan L, Li P et al (2009) All-trans retinoic acid promotes neural lineage entry by pluripotent embryonic stem cells via multiple pathways. BMC Cell Biol 10:57. doi:10.1186/1471-2121-10-57
Wiederschain D, Susan W, Chen L et al (2009) Single-vector inducible lentiviral RNAi system for oncology target validation. Cell Cycle 8:498–504. doi:10.4161/cc.8.3.7701
Khodeer S, Era T (2017) Identifying the biphasic role of calcineurin/NFAT signaling enables replacement of Sox2 in somatic cell reprogramming: calcineurin/NFAT signaling in reprogramming. Stem Cells. doi:10.1002/stem.2572
Lo Nigro A, de Jaime-Soguero A, Khoueiry R et al (2017) PDGFRα+ cells in embryonic stem cell cultures represent the in vitro equivalent of the pre-implantation primitive endoderm precursors. Stem Cell Reports 8:318–333. doi:10.1016/j.stemcr.2016.12.010
Loh KM, Lim B (2011) A precarious balance: pluripotency factors as lineage specifiers. Cell Stem Cell 8:363–369. doi:10.1016/j.stem.2011.03.013
Martínez-Martínez S, Redondo JM (2004) Inhibitors of the calcineurin/NFAT pathway. Curr Med Chem 11:997–1007
Tanaka H, Kuroda A, Marusawa H et al (1987) Structure of FK506, a novel immunosuppressant isolated from Streptomyces. J Am Chem Soc 109:5031–5033. doi:10.1021/ja00250a050
García JEL, Rodríguez FM, López AJ et al (1998) Effect of cyclosporin A on inflammatory cytokine production by human alveolar macrophages. Respir Med 92:722–728. doi:10.1016/S0954-6111(98)90002-6
Borsini A, Zunszain PA, Thuret S, Pariante CM (2015) The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci 38:145–157. doi:10.1016/j.tins.2014.12.006
Van Wagoner NJ, Oh JW, Repovic P, Benveniste EN (1999) Interleukin-6 (IL-6) production by astrocytes: autocrine regulation by IL-6 and the soluble IL-6 receptor. J Neurosci 19:5236–5244
Erta M, Quintana A, Hidalgo J (2012) Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 8:1254–1266. doi:10.7150/ijbs.4679
Powles RL, Clink HM, Spence D et al (1980) Cyclosporin A to prevent graft-versus-host disease in man after allogeneic bone-marrow transplantation. Lancet 315:327–329. doi:10.1016/S0140-6736(80)90881-8
Solomon SR, Nakamura R, Read EJ et al (2003) Cyclosporine is required to prevent severe acute GVHD following T-cell-depleted peripheral blood stem cell transplantation. Bone Marrow Transplant 31:783–788. doi:10.1038/sj.bmt.1703928
Altman RD, Schiff M, Kopp EJ (1999) Cyclosporine A in rheumatoid arthritis: randomized, placebo controlled dose finding study. J Rheumatol 26:2102–2109
Ellis CN, Fradin MS, Messana JM et al (1991) Cyclosporine for plaque-type psoriasis: results of a multidose, double-blind trial. N Engl J Med 324:277–284. doi:10.1056/NEJM199101313240501
Ptachcinski RJ, Venkataramanan R, Burckart GJ (1986) Clinical Pharmacokinetics of Cyclosporin: Clinical Pharmacokinetics 11:107–132. doi:10.2165/00003088-198611020-00002
Fujiwara M, Yan P, Otsuji TG et al (2011) Induction and enhancement of cardiac cell differentiation from mouse and human induced pluripotent stem cells with Cyclosporin-A. PLoS One 6:e16734. doi:10.1371/journal.pone.0016734
Sun M, Liao B, Tao Y et al (2016) Calcineurin-NFAT signaling controls somatic cell reprogramming in a stage-dependent manner: calcineurin-NFAT signaling in reprogramming. J Cell Physiol 231:1151–1162. doi:10.1002/jcp.25212
Sato N, Meijer L, Skaltsounis L et al (2004) Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 10:55–63. doi:10.1038/nm979
Qyang Y, Martin-Puig S, Chiravuri M et al (2007) The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/β-catenin pathway. Cell Stem Cell 1:165–179. doi:10.1016/j.stem.2007.05.018
Tarafdar A, Dobbin E, Corrigan P et al (2013) Canonical Wnt signaling promotes early hematopoietic progenitor formation and erythroid specification during embryonic stem cell differentiation. PLoS One 8:e81030. doi:10.1371/journal.pone.0081030
Compagnucci C, Nizzardo M, Corti S et al (2014) In vitro neurogenesis: development and functional implications of iPSC technology. Cell Mol Life Sci 71:1623–1639. doi:10.1007/s00018-013-1511-1
Cho A, Tang Y, Davila J et al (2014) Calcineurin signaling regulates neural induction through antagonizing the BMP pathway. Neuron 82:109–124. doi:10.1016/j.neuron.2014.02.015
Jacobs S, Lie DC, DeCicco KL et al (2006) Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci 103:3902–3907. doi:10.1073/pnas.0511294103
Janesick A, Wu SC, Blumberg B (2015) Retinoic acid signaling and neuronal differentiation. Cell Mol Life Sci 72:1559–1576. doi:10.1007/s00018-014-1815-9
Chow A, Morshead CM (2016) Cyclosporin A enhances neurogenesis in the dentate gyrus of the hippocampus. Stem Cell Res 16:79–87. doi:10.1016/j.scr.2015.12.007
Vilar M, Mira H (2016) Regulation of neurogenesis by neurotrophins during adulthood: expected and unexpected roles. Front Neurosci. doi:10.3389/fnins.2016.00026
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660. doi:10.1016/j.cell.2008.01.033
Satoh T, Nakamura S, Taga T et al (1988) Induction of neuronal differentiation in PC12 cells by B-cell stimulatory factor 2/interleukin 6. Mol Cell Biol 8:3546–3549
März P, Herget T, Lang E et al (1997) Activation of gp130 by IL-6/soluble IL-6 receptor induces neuronal differentiation. Eur J Neurosci 9:2765–2773
Schafer K-H, Mestres P, Marz P, Rose-John S (1999) The IL-6/sIL-6R fusion protein hyper-IL-6 promotes neurite outgrowth and neuron survival in cultured enteric neurons. J Interf Cytokine Res 19:527–532. doi:10.1089/107999099313974
Kahn MA, de Vellis J (1994) Regulation of an oligodendrocyte progenitor cell line by the interleukin-6 family of cytokines. Glia 12:87–98. doi:10.1002/glia.440120202
Nakanishi M, Niidome T, Matsuda S et al (2007) Microglia-derived interleukin-6 and leukemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells: microglia and NSPC differentiation. Eur J Neurosci 25:649–658. doi:10.1111/j.1460-9568.2007.05309.x
Maeda K, Mehta H, Drevets DA, Coggeshall KM (2010) IL-6 increases B-cell IgG production in a feed-forward proinflammatory mechanism to skew hematopoiesis and elevate myeloid production. Blood 115:4699–4706. doi:10.1182/blood-2009-07-230631
Kishimoto T, Akira S, Narazaki M, Taga T (1995) Interleukin-6 family of cytokines and gp130. Blood 86:1243–1254
Murayama K, Sawamura M, Murakami H et al (1994) FK506 and cyclosporin A enhance IL-6 production in monocytes: a single-cell assay. Mediat Inflamm 3:375–380. doi:10.1155/S0962935194000529
Acknowledgements
We would like to thank AK lab members for critical review of all the data. We thank Prof. Catherine Verfaillie for the generous gift of Oct4-GFP iPSCs. This work was supported by grants from CSIR [no. 27(0294)/13/EMRII] and intramural funding from SORM, Manipal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Fig. 1
Pluripotency and lineage expression analysis of ESCs treated with CsA. a Semi –quantitative PCR analysis of Src in mESCs in presence and absence of CsA b Semi-quantitative PCR showing the expression of pluripotency genes in mESCs in absence and presence of different concentrations of CsA. c Realtime PCR for lineage specific markers in mESCs treated with RA and CsA and withdrawal of LIF. d qPCR results showing lineage specific marker expression in EBs derived from mESCs with Day 3 to Day 14 CsA treatment. (GIF 302 kb).
Supplementary Fig. 2
CsA resuces OCT4-GFP expression and maintains pluripotency of miPSCs. a Flowcytometric analysis and b quantification of percent bright GFP positive miPSCs expressing OCT4-GFP. Single asterisk indicates p < 0.05 vs cells cultured in presence of LIF. (GIF 29 kb).
Supplementary Fig. 3
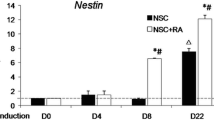
Differentiation of mESCs using N2B27 and RA demarcates early and late differentiation events. a Gene expression analysis and b immunofluorescence of of neural progenitor genes in the early differentiation event. c Transcript analysis and immunofluorescence analysis (d) of neurons derived from mPSCs during late differentiation Data is representative of three independent experiments and presented as mean ± SEM. Scale bar represents 100 μm. (GIF 82 kb).
Supplementary Fig. 4
CsA inhibits nuclear translocation of NFATC3. a Immunoblot depiciting NFATC3 levels in the nuclear extracts of neurons differentiated from mESCs with and without CsA; purity of nuclear extracts assessed by presence of Nulceolin and absence of ß-actin. (GIF 6 kb).
Supplementary Fig. 5
KnockdownPpp3r1 enhances neural gene expression in mESCs. a Gene expression analysis of Ppp3r1 in Scrambled and Ppp3r1 shRNA in mESCs. b Neural gene expression of mESC scramble and Ppp3r1knockdown cells. Data is representative of three independent experiments and presented as mean ± SEM. *p ≤ 0.05 vs scrambled control. (GIF 12 kb).
Supplementary Fig. 6
Blocking of Cn-NFAT pathway is essential for IL-6 expression in iPSCs. a Transcript analysis of miPSCs derived embryoid bodies in the presence and absence of CsA for IL-6 expression. b mRNA expression of interleukin genes in miPSCs transduced with PPP3r1 shRNA and scrambled control and differentiated to neural lineage. Data is representative of three independent experiments and presented as mean ± SEM. *p < 0.05, **p < 0.01 vs scrambled control. c qPCR analysis of interleukin genes in miPSCs differentiated to neural lineage in the presence and absence of FK506. (GIF 28 kb).
Supplementary Table 1
(TIFF 18 kb).
Supplementary Table 2
(TIFF 12 kb).
Rights and permissions
About this article
Cite this article
Ashwini, A., Naganur, S.S., Smitha, B. et al. Cyclosporine A-Mediated IL-6 Expression Promotes Neural Induction in Pluripotent Stem Cells. Mol Neurobiol 55, 4267–4279 (2018). https://doi.org/10.1007/s12035-017-0633-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0633-0