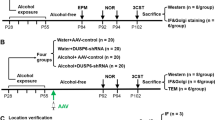
Abstract
In the young population, binge drinking is a pattern of problematic alcohol consumption, characterized by a short period of heavy drinking followed by abstinence which is frequently repeated over time. This drinking pattern is associated with mental problems, use of other drugs, and an increased risk of excessive alcohol intake during adulthood. However, little is known about the effects of binge drinking on brain function in adolescents and its neurobiological impact during the adulthood. In the present study, we evaluated the effects of alcohol on hippocampal memory, synaptic plasticity, and mitochondrial function in adolescent rats after a binge drinking episode in vivo. These effects were analyzed at 1, 3, or 7 weeks post alcohol exposure. Our results showed that binge-like ethanol pre-treated (BEP) rats exhibited early alterations in learning and memory tests accompanied by an impairment of synaptic plasticity that was total and partially compensated, respectively. These changes could be attributed to a rapid increase in oxidative damage and a late inflammatory response induced by post ethanol exposure. Additionally, BEP alters the regulation of mitochondrial dynamics and modifies the expression of mitochondrial permeability transition pore (mPTP) components, such as cyclophilin D (Cyp-D) and the voltage-dependent anion channel (VDAC). These mitochondrial structural changes result in the impairment of mitochondrial bioenergetics, decreasing ATP production progressively until adulthood. These results strongly suggest that teenage alcohol binge drinking impairs the function of the adult hippocampus including memory and synaptic plasticity as a consequence of the mitochondrial damage induced by alcohol and that the recovery of hippocampal function could implicate the activation of alternative pathways that fail to reestablish mitochondrial function.









Similar content being viewed by others
Change history
15 August 2018
The authors declare that the original version of this article contained a mistake in the data of the Figure 2, particularly in the LTP data.
References
Koob GF, Le Moal M (2005) Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci 8(11):1442–1444. doi:10.1038/nn1105-1442
Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF (2008) Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res 32(12):2149–2160. doi:10.1111/j.1530-0277.2008.00806.x
Kirby T, Barry AE (2012) Alcohol as a gateway drug: a study of US 12th graders. J Sch Health 82(8):371–379. doi:10.1111/j.1746-1561.2012.00712.x
SAMHSA (2007) Substance abuse and mental health services administration. National survey on drug use and health: national findings. SAMHSA, Rockville
Merrill JE, Carey KB (2016) Drinking over the lifespan: focus on college ages. Alcohol Res 38(1):103–114
Briones TL, Woods J (2013) Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience 254:324–334. doi:10.1016/j.neuroscience.2013.09.031
McBride O, Cheng HG (2011) Exploring the emergence of alcohol use disorder symptoms in the two years after onset of drinking: findings from the National Surveys on drug use and health. Addiction 106(3):555–563. doi:10.1111/j.1360-0443.2010.03242.x
Wilcox MV, Cuzon Carlson VC, Sherazee N, Sprow GM, Bock R, Thiele TE, Lovinger DM, Alvarez VA (2014) Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology 39(3):579–594. doi:10.1038/npp.2013.230
DeWit DJ, Adlaf EM, Offord DR, Ogborne AC (2000) Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry 157(5):745–750. doi:10.1176/appi.ajp.157.5.745
Spear L (2000) Modeling adolescent development and alcohol use in animals. Alcohol Res Health 24(2):115–123
Dahl RE (2004) Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci 1021:1–22. doi:10.1196/annals.1308.0011021/1/1
Slawecki CJ, Thorsell A, Ehlers CL (2004) Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models. Ann N Y Acad Sci 1021:448–452. doi:10.1196/annals.1308.0621021/1/448
White AM, Matthews DB, Best PJ (2000) Ethanol, memory, and hippocampal function: a review of recent findings. Hippocampus 10(1):88–93. doi:10.1002/(SICI)1098-1063(2000)10:1<88::AID-HIPO10>3.0.CO;2-L
Obernier JA, Bouldin TW, Crews FT (2002) Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res 26(4):547–557
Rimessi A, Previati M, Nigro F, Wieckowski MR, Pinton P (2016) Mitochondrial reactive oxygen species and inflammation: molecular mechanisms, diseases and promising therapies. Int J Biochem Cell Biol. doi:10.1016/j.biocel.2016.06.015
Pelletier M, Lepow TS, Billingham LK, Murphy MP, Siegel RM (2012) New tricks from an old dog: mitochondrial redox signaling in cellular inflammation. Semin Immunol 24(6):384–392. doi:10.1016/j.smim.2013.01.002
Picard M, McEwen BS (2014) Mitochondria impact brain function and cognition. Proc Natl Acad Sci U S A 111(1):7–8. doi:10.1073/pnas.1321881111
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417(1):1–13. doi:10.1042/BJ20081386
Levy M, Faas GC, Saggau P, Craigen WJ, Sweatt JD (2003) Mitochondrial regulation of synaptic plasticity in the hippocampus. J Biol Chem 278(20):17727–17734. doi:10.1074/jbc.M212878200
Reddy VD, Padmavathi P, Kavitha G, Saradamma B, Varadacharyulu N (2013) Alcohol-induced oxidative/nitrosative stress alters brain mitochondrial membrane properties. Mol Cell Biochem 375(1–2):39–47. doi:10.1007/s11010-012-1526-1
Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C (2007) Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci 25(2):541–550. doi:10.1111/j.1460-9568.2006.05298.x
Tapia-Rojas C, Lindsay CB, Montecinos-Oliva C, Arrazola MS, Retamales RM, Bunout D, Hirsch S, Inestrosa NC (2015) Is l-methionine a trigger factor for Alzheimer's-like neurodegeneration?: changes in abeta oligomers, tau phosphorylation, synaptic proteins, Wnt signaling and behavioral impairment in wild-type mice. Mol Neurodegener 10:62. doi:10.1186/s13024-015-0057-0
Tapia-Rojas C, Schuller A, Lindsay CB, Ureta RC, Mejias-Reyes C, Hancke J, Melo F, Inestrosa NC (2015) Andrographolide activates the canonical Wnt signalling pathway by a mechanism that implicates the non-ATP competitive inhibition of GSK-3beta: autoregulation of GSK-3beta in vivo. Biochem J 466(2):415–430. doi:10.1042/BJ20140207
Crews FT, Braun CJ, Hoplight B, Switzer RC 3rd, Knapp DJ (2000) Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res 24(11):1712–1723
Guerri C, Pascual M (2010) Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol 44(1):15–26. doi:10.1016/j.alcohol.2009.10.003
Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L et al (2000) A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature 408(6815):975–979. doi:10.1038/35050103
McCool BA (2011) Ethanol modulation of synaptic plasticity. Neuropharmacology 61(7):1097–1108. doi:10.1016/j.neuropharm.2010.12.028
Serrano FG, Tapia-Rojas C, Carvajal FJ, Hancke J, Cerpa W, Inestrosa NC (2014) Andrographolide reduces cognitive impairment in young and mature AbetaPPswe/PS-1 mice. Mol Neurodegener 9:61. doi:10.1186/1750-1326-9-61
Jarvenpaa T, Rinne JO, Koskenvuo M, Raiha I, Kaprio J (2005) Binge drinking in midlife and dementia risk. Epidemiology 16(6):766–771
Yakovleva T, Bazov I, Watanabe H, Hauser KF, Bakalkin G (2011) Transcriptional control of maladaptive and protective responses in alcoholics: a role of the NF-kappaB system. Brain Behav Immun 25(Suppl 1):S29–S38. doi:10.1016/j.bbi.2010.12.019
Blanco AM, Pascual M, Valles SL, Guerri C (2004) Ethanol-induced iNOS and COX-2 expression in cultured astrocytes via NF-kappa B. Neuroreport 15(4):681–685
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):a001651. doi:10.1101/cshperspect.a001651
Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW (2007) Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem 55(7):687–700. doi:10.1369/jhc.6A7156.2007
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35. doi:10.1007/s00401-009-0619-8
Manfredi G, Beal MF (2007) Merging mitochondria for neuronal survival. Nat Med 13(10):1140–1141. doi:10.1038/nm1007-1140
Cederbaum AI (2012) Alcohol metabolism. Clin Liver Dis 16(4):667–685. doi:10.1016/j.cld.2012.08.002
Das S, Hajnoczky N, Antony AN, Csordas G, Gaspers LD, Clemens DL, Hoek JB, Hajnoczky G (2012) Mitochondrial morphology and dynamics in hepatocytes from normal and ethanol-fed rats. Pflugers Arch 464(1):101–109. doi:10.1007/s00424-012-1100-4
Chan DC (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125(7):1241–1252. doi:10.1016/j.cell.2006.06.010
Twig G, Hyde B, Shirihai OS (2008) Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta 1777(9):1092–1097. doi:10.1016/j.bbabio.2008.05.001
Cheng A, Hou Y, Mattson MP (2010) Mitochondria and neuroplasticity. ASN Neuro 2(5):e00045. doi:10.1042/AN20100019
Mattson MP, Gleichmann M, Cheng A (2008) Mitochondria in neuroplasticity and neurological disorders. Neuron 60(5):748–766. doi:10.1016/j.neuron.2008.10.010
Liesa M, Palacin M, Zorzano A (2009) Mitochondrial dynamics in mammalian health and disease. Physiol Rev 89(3):799–845. doi:10.1152/physrev.00030.2008
Olichon A, Elachouri G, Baricault L, Delettre C, Belenguer P, Lenaers G (2007) OPA1 alternate splicing uncouples an evolutionary conserved function in mitochondrial fusion from a vertebrate restricted function in apoptosis. Cell Death Differ 14(4):682–692. doi:10.1038/sj.cdd.4402048
Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, Langer T (2014) The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol 204(6):919–929. doi:10.1083/jcb.201308006
Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29(28):9090–9103. doi:10.1523/JNEUROSCI.1357-09.2009
Westermann B (2012) Bioenergetic role of mitochondrial fusion and fission. Biochim Biophys Acta 1817(10):1833–1838. doi:10.1016/j.bbabio.2012.02.033
Halestrap AP (2009) What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 46(6):821–831. doi:10.1016/j.yjmcc.2009.02.021
Gutierrez-Aguilar M, Baines CP (2015) Structural mechanisms of cyclophilin D-dependent control of the mitochondrial permeability transition pore. Biochim Biophys Acta 1850(10):2041–2047. doi:10.1016/j.bbagen.2014.11.009
Narasimhan M, Mahimainathan L, Rathinam ML, Riar AK, Henderson GI (2011) Overexpression of Nrf2 protects cerebral cortical neurons from ethanol-induced apoptotic death. Mol Pharmacol 80(6):988–999. doi:10.1124/mol.111.073262
Dinkova-Kostova AT, Abramov AY (2015) The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med 88(Pt B):179–188. doi:10.1016/j.freeradbiomed.2015.04.036
Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, Chen W (2008) Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U S A 105(17):6409–6414. doi:10.1073/pnas.0710766105
Detmer SA, Chan DC (2007) Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8(11):870–879. doi:10.1038/nrm2275
Schulteis G, Archer C, Tapert SF, Frank LR (2008) Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol 42(6):459–467. doi:10.1016/j.alcohol.2008.05.002
Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF (2010) Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction 106(3):564–573. doi:10.1111/j.1360-0443.2010.03197.x
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1(2):848–858. doi:10.1038/nprot.2006.116
Jeneson A, Kirwan CB, Hopkins RO, Wixted JT, Squire LR (2010) Recognition memory and the hippocampus: a test of the hippocampal contribution to recollection and familiarity. Learn Mem 17(1):63–70. doi:10.1101/lm.1546110
Prendergast MA, Harris BR, Mullholland PJ, Blanchard JA 2nd, Gibson DA, Holley RC, Littleton JM (2004) Hippocampal CA1 region neurodegeneration produced by ethanol withdrawal requires activation of intrinsic polysynaptic hippocampal pathways and function of N-methyl-d-aspartate receptors. Neuroscience 124(4):869–877. doi:10.1016/j.neuroscience.2003.12.013
Krystal JH, Petrakis IL, Mason G, Trevisan L, D'Souza DC (2003) N-Methyl-d-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther 99(1):79–94
Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, Adams LJ, Vengeliene V et al (2010) Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry 67(10):1069–1077. doi:10.1001/archgenpsychiatry.2010.125
Ron D (2004) Signaling cascades regulating NMDA receptor sensitivity to ethanol. Neuroscientist 10(4):325–336. doi:10.1177/1073858404263516
Woodward JJ (2000) Ethanol and NMDA receptor signaling. Crit Rev Neurobiol 14(1):69–89
Moykkynen T, Korpi ER, Lovinger DM (2003) Ethanol inhibits alpha-amino-3-hydyroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor function in central nervous system neurons by stabilizing desensitization. J Pharmacol Exp Ther 306(2):546–555. doi:10.1124/jpet.103.050666jpet.103.050666
Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H et al (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62(3):405–496. doi:10.1124/pr.109.002451
Granger AJ, Nicoll RA (2014) Expression mechanisms underlying long-term potentiation: a postsynaptic view, 10 years on. Philos Trans R Soc Lond Ser B Biol Sci 369(1633):20130136. doi:10.1098/rstb.2013.0136
Lee KF, Soares C, Beique JC (2012) Examining form and function of dendritic spines. Neural Plast 2012:704103. doi:10.1155/2012/704103
Zorumski CF, Mennerick S, Izumi Y (2014) Acute and chronic effects of ethanol on learning-related synaptic plasticity. Alcohol 48(1):1–17. doi:10.1016/j.alcohol.2013.09.045
Valenzuela CF (1997) Alcohol and neurotransmitter interactions. Alcohol Health Res World 21(2):144–148
Zou JY, Crews FT (2005) TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res 1034(1–2):11–24. doi:10.1016/j.brainres.2004.11.014
Zipp F, Aktas O (2006) The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci 29(9):518–527. doi:10.1016/j.tins.2006.07.006
Luikart BW, Schnell E, Washburn EK, Bensen AL, Tovar KR, Westbrook GL (2011) Pten knockdown in vivo increases excitatory drive onto dentate granule cells. J Neurosci 31(11):4345–4354. doi:10.1523/JNEUROSCI.0061-11.2011
Jung ME (2015) Alcohol withdrawal and cerebellar mitochondria. Cerebellum 14(4):421–437. doi:10.1007/s12311-014-0598-8
Kann O, Kovacs R (2007) Mitochondria and neuronal activity. Am J Physiol Cell Physiol 292(2):C641–C657. doi:10.1152/ajpcell.00222.2006
Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E (2008) Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9(7):505–518. doi:10.1038/nrn2417
Archer SL (2013) Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. N Engl J Med 369(23):2236–2251. doi:10.1056/NEJMra1215233
Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1(4):515–525
Bernhardt D, Muller M, Reichert AS, Osiewacz HD (2015) Simultaneous impairment of mitochondrial fission and fusion reduces mitophagy and shortens replicative lifespan. Sci Rep 5:7885. doi:10.1038/srep07885
Crews FT, Nixon K (2009) Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol 44(2):115–127. doi:10.1093/alcalc/agn079
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552(Pt 2):335–344. doi:10.1113/jphysiol.2003.049478
Keynes RG, Garthwaite J (2004) Nitric oxide and its role in ischaemic brain injury. Curr Mol Med 4(2):179–191
Jin M, Ande A, Kumar A, Kumar S (2013) Regulation of cytochrome P450 2e1 expression by ethanol: role of oxidative stress-mediated pkc/jnk/sp1 pathway. Cell Death Dis 4:e554. doi:10.1038/cddis.2013.78
Montoliu C, Valles S, Renau-Piqueras J, Guerri C (1994) Ethanol-induced oxygen radical formation and lipid peroxidation in rat brain: effect of chronic alcohol consumption. J Neurochem 63(5):1855–1862
Lopez-Armada MJ, Riveiro-Naveira RR, Vaamonde-Garcia C, Valcarcel-Ares MN (2013) Mitochondrial dysfunction and the inflammatory response. Mitochondrion 13(2):106–118. doi:10.1016/j.mito.2013.01.003
Tschopp J (2011) Mitochondria: sovereign of inflammation? Eur J Immunol 41(5):1196–1202. doi:10.1002/eji.201141436
Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL (2005) Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J Pharmacol Exp Ther 314(2):780–788. doi:10.1124/jpet.105.085779
Friberg H, Wieloch T (2002) Mitochondrial permeability transition in acute neurodegeneration. Biochimie 84(2–3):241–250
Tanaka D, Nakada K, Takao K, Ogasawara E, Kasahara A, Sato A, Yonekawa H, Miyakawa T et al (2008) Normal mitochondrial respiratory function is essential for spatial remote memory in mice. Mol Brain 1:21. doi:10.1186/1756-6606-1-21
Mattson M (2004) Pathways towards and away from Alzheimer’s disease. Nature 430(7000):631–639
Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG et al (2007) Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem 100(5):1375–1386. doi:10.1111/j.1471-4159.2006.04327.x
Acknowledgements
This work was supported by FONDECYT: 1140968 and 1170441 (to RAQ), 11121206 (to WC), 11121133 and 1160710 (to JAO), 11150308 (to JML), Proyecto de Cooperación Internacional (PCI), BMBF 20150065 (to WC), and Anillo ACT1411 (to RAQ, WC, JAO, and JML).
Author information
Authors and Affiliations
Contributions
CTR, RAQ, WC, JAO, and JML designed and coordinated the study. CTR, FJC, RMG, and CA conducted most of the experiments and performed the statistical analyses. CTR, WC, and RAQ wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Prof. Rodrigo A. Quintanilla is the first corresponding author.
Electronic supplementary material
Fig. S1
Ethanol binge-like administration induces oxidative damage in the brain of adolescent rats. Representative images from immunofluorescence of the CA3 region of the hippocampus obtained from animals at three different time points: 1 week after injections (n = 3), 3 weeks after injections (n = 3) and 7 weeks after injections (n = 3). Hoechst staining was used to identify the nuclei, 8-hydroxyguanine (DNA damage) immunoreactivity and merge images are shown. Quantification of images shows a comparison between the 3 different times after ethanol treatment. There was a decrease in 8-hydroxyguanine immunoreactivity 1 week after ethanol injections, and there was no difference between the control and ethanol group at the other measured times. *p < 0.05; n.s not significant. Bars represent the mean ± SEM. *p < 0.05. (GIF 2.0 mb)
Fig. S2
Ethanol binge-like administration not alters the mitochondrial mass in the brain of adolescent rats. Representative images obtained from SP or BEP rats. 25 μM coronal slices of unfixed tissue were incubated with MitoTracker Green FM for 45 min a 37 °C. Fluoroshield Mounting Medium with DAPI was also used. The relative intensity shows no significant differences between SP and BEP rats in CA1, CA3 and Dentate Gyrus. The number of cells per field was similar in both control and treated rats. High Resolution Image (GIF 1.45 mb)
Fig. S3
Ethanol binge-like administration not induces changes in the number of cells in the hippocampus of treated rats. Representative images of Nissl stain to evaluate the cell density in the CA1, CA3 and DG region from the hippocampus. Analysis reveals similar density of cells in both SP and BEP rats in all regions measured.(GIF 1.88 mb)
Table S1
Ethanol binge-like administration no induces changes in the locomotor activity. Swimming rate in the MWM, average speed in the NOR and average travel speed in the SI task in both SP and BEP rats. No significant differences were observed between the experimental groups. (GIF 275 kb)
Rights and permissions
About this article
Cite this article
Tapia-Rojas, C., Carvajal, F.J., Mira, R.G. et al. Adolescent Binge Alcohol Exposure Affects the Brain Function Through Mitochondrial Impairment. Mol Neurobiol 55, 4473–4491 (2018). https://doi.org/10.1007/s12035-017-0613-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0613-4