Abstract
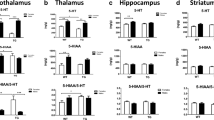
Trisomy 21 (T21) or Down syndrome (DS) is the most common genetic disorder associated with intellectual disability and affects around 5 million persons worldwide. Neuroanatomical phenotypes associated with T21 include slight reduction of brain size and weight, abnormalities in several brain areas including spines dysgenesis, dendritic morphogenesis, and early neuroanatomical characteristics of Alzheimer’s disease. Monoamine neurotransmitters are involved in dendrites development, functioning of synapses, memory consolidation, and their levels measured in the cerebrospinal fluid, blood, or brain areas that are modified in individuals with T21. DYRK1A is one of the recognized key genes that could explain some of the deficits present in individuals with T21. We investigated by high-performance liquid chromatography with electrochemical detection the contents and processing of monoamines neurotransmitters in four brain areas of female and male transgenic mice for the Dyrk1a gene (mBactgDyrk1a). DYRK1A overexpression induced dramatic deficits in the serotonin contents of the four brain areas tested and major deficits in dopamine and adrenaline contents especially in the hypothalamus. These results suggest that DYRK1A overexpression might be associated with the modification of monoamines content found in individuals with T21 and reinforce the interest to target the level of DYRK1A expression as a therapeutic approach for persons with T21.






Similar content being viewed by others
References
Pueschel SM (1990) Clinical aspects of down syndrome from infancy to adulthood. Am J Med Genet Suppl 7:52–56
Vicari S (2006) Motor development and neuropsychological patterns in persons with down syndrome. Behav Genet 36:365–364
Suetsugu M, Mehraein P (1980) Spines distribution along the apical dendrites of the pyramidal neurons in Down’s syndrome. Quantitative Golgi study Acta Neuropathol 50:207–210
Coyle JT, Oster-Granite ML, Gearhart JD (1986) The neurobiologic consequences of down syndrome. Brain Res Bull 16:773–787
Lott IT (2012) Neurological phenotypes for down syndrome across the life span. Prog Brain Res 197:101–121
Hartley D, Blumenthal T, Carrillo M et al (2015) Down syndrome and Alzheimer’s disease: common pathways, common goals. Alzheimers Dement 11:700–709
Eisenhofer G, Kopin IJ, Goldstein DS (2004) Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56:331–349
Kehagia AA, Murray GK, Robbins TW (2010) Learning and cognitive flexibility: fronto-striatal function and monoaminergic modulation. Curr Opin Neurobiol 20:199–204
Brodie BB, Shore PA (1957) A concept for a role of serotonin and norepinephrine as chemical mediators in the brain. Ann N Y Acad Sci 66:631–642
Sodhi M, Sanders-Bush E (2004) Serotonin and brain development. Int. Rev Neurobiol 59:111–174
Trakhtenberg EF, Goldberg JL (2012) The role of serotonin in axon and dendrite growth. Int Rev Neurobiol 106:105–126
Gaspar P, Cases O, Maroteaux L (2003) The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci 4:1002–1012
Narboux-Nême N, Angenard G, Mosienko V et al (2013) Postnatal growth defects in mice with constitutive depletion of central serotonin. ACS Chem Neurosci 4:171–181
Snyder SH (2011) What dopamine does in the brain. PNAS 108:18869–18871
Tritsch NX, Sabatini BL (2012) Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76:33–50
Xing B, Li YC, Gao WJ (2016) Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain Res 1641:217–233
Cools R, Nakamura K, Daw ND (2011) Serotonin and dopamine: unifying affective, activational and decision functions. Neuropsychopharmacol Rev 36:98–113
Okado N, Narita M, Narita N (2001) A biogenic amine-synapse mechanism for mental retardation and developmental disabilities. Brain and Development 23(Suppl 1):S11–S15
Rodríguez JJ, Noristani HN, Verkhratsky A (2012) The serotonergic system in ageing and Alzheimer’s disease. Prog Neurobiol 99:15–41
Muller CL, Anacker AMJ, Veenstra-Vanderweele J (2016) The serotonin system in autism spectrum disorder: from biomarkers to animal models. Neurosci 321:24–41
Matias S, Lottem E, Dugué GP, Mainen ZF (2017) Activity patterns of serotonin neurons underlying cognitive flexibility. Elife : 6
Tu JH, Zellweger H (1965) Blood-serotonin deficiency in Down’s syndrome. Lancet 2:715–716
Boullin DJ, O’Brien RA (1971) Abnormalities of 5-Hydroxytryptamine uptake and binding by blood platelets from children with Down’s syndrome. J Physiol 212:287–297
Lott IT, Chase TN, Murphy DL (1972) Down’s syndrome: transport, storage, and metabolism of serotonin in blood platelets. Pediatr Res 6:730–735
Mann DM, Lincoln J, Yates PO, Brennan CM (1980) Monoamine metabolism in down syndrome. Lancet 2:1366–1367
Wetterberg L, Gustavson KH, Backström M, Ross SB, Fröden O (1972) Low dopamine-beta-hydroxylase activity in Down’s syndrome. Clin Genet 3:152–153
Risser D, Lubec G, Cairns N, Herrera-Marschitz M (1997) Excitatory amino acids and monoamines in parahippocampal gyrus and frontal cortical pole of adults with down syndrome. Life Sci 60:1231–1237
Seidl R, Kaehler ST, Prast H et al (1999) Serotonin (5-HT) in brains of adult patients with down syndrome. J Neural Trans Sup 57:221–232
Whittle N, Sartori SB, Dierssen M, Lubec G, Singewald N (2007) Fetal down syndrome brains exhibit aberrant levels of neurotransmitters critical for normal brain development. Pediatrics 120:e1465–e1471
Davisson MT, Schmidt C (1993) Reeves RH et al. Segmental trisomy as a mouse model for Down syndrome Prog Clin Biol Res 384:117–133
Gardiner K, Herault Y, Lott IT, Antonarakis SE, Reeves RH, Dierssen M (2010) Down syndrome: from understanding the neurobiology to therapy. J Neurosci 3:14943–14945
Creau N (2012) Molecular and cellular alterations in Down syndrome: toward the identification of targets for therapeutics. Neural Plast 2012:171639
Gotti S, Caricati E, Panzica G (2011) Alterations of brain circuits in down syndrome murine models. J Chem Neuroanat 42:317–326
Rueda N, Flórez J, Martínez-Cué C (2012) Mouse models of down syndrome as a tool to unravel the causes of mental disabilities. Neural Plast 2012:584071
Phillips C, Fahimi A, Das D, Mojabi FS, Ponnusamy R, Salehi A (2016) Noradrenergic system in down syndrome and Alzheimer’s disease a target for therapy. Cur Alz Res 13:68–83
Das D, Phillips C, Hsieh W et al (2014) Neurotransmitter-based strategies for the treatment of cognitive dysfunction in down syndrome. Prog Neuro-Psychopharmacol Biol Psychiatry 54:140–148
Begenesic T, Baroncelli L, Sansevero G, Milanese M et al (2014) Fluoxetine in adulthood normalizes GABA release and rescues hippocampal synaptic plasticity and spatial memory in a mouse model of down syndrome. Neurobiol Dis 63:12–19
Tejedor F, Zhu XR, Kaltenbach E, Ackermann A et al (1995) Minibrain: a new protein kinase family involved in post embryonic neurogenesis in Drosophila. Neuron 14:287–301
Guimera J, Casas C, Estivill X, Pritchard M (1999) Human minibrain homologue (MNBH/DYRK1): characterization, alternative splicing, differential tissue expression, and overexpression in Down syndrome. Genomics 57:407–418
Dowjat WK, Adayev T, Kuchna I et al (2007) Trisomy-driven overexpression of DYRK1A kinase in the brain of subjects with Down syndrome. Neurosci Lett 413:77–81
Kay LJ, Smulders-Srinivasan TK, Soundararajan M (2016) Understanding the multifaceted role of human down syndrome kinase DYRK1A. Adv Protein Chem Struct Biol 105:127–171
Guedj F, Lopes Pereira P, Najas S et al (2012) DYRK1A: a master regulatory protein controlling brain growth. Neurobiol Dis 46:190–203
Duchon A, Herault Y (2016) DYRK1A, a dosage-sensitive gene involved in neurodevelopmental disorders is a target for drug development in Down syndrome. Front Behav Neurosci 10:104–117
Kim H, Lee KS, Kim AK, Choi M et al (2016) A chemical with proven clinical safety rescues Down-syndrome-related phenotypes in through DYRK1A inhibition. Dis Model Mech 9:839–848
Souchet B, Guedj F, Sahún I et al (2014) Excitation/inhibition balance and learning are modified by Dyrk1a gene dosage. Neurobiol Dis 69:65–75
Souchet B, Guedj F, Penke-Verdier Z et al (2015) Pharmacological correction of excitation/inhibition imbalance in Down syndrome mouse models. Front Behav Neurosci 9:1–11
Bronicki LM, Redin C, Drunat S et al (2015) Ten new cases further delineate the syndromic intellectual disability phenotype caused by mutations in DYRK1A. Eur J Hum Genet 23:1482–1487
Dang T, Duan WY, Yu B, Tong DL et al (2017) Autism-associated Dyrk1a truncation mutants impair neuronal dendritic and spine growth and interfere with postnatal cortical development. Mol Psychiatry 00:1–12
Cosgrove KP, Mazure CM, Staley JK (2007) Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 62:847–855
McCarthy MM (2016) Multifaceted origins of sex differences in the brain. Phil Trans R Soc B 371:20150106
Hirata-Fukae C, Li HF, Hoe HS et al (2008) Females exhibit more extensive amyloid, but not tau, pathology in an Alzheimer transgenic model. Brain Res 1216:92–103
Simpson J, Ryan C, Curley A, Mulcaire J, Kelly JP (2012) Sex differences in baseline and drug-induced behavioural responses in classical behavioural tests. Prog Neuro-Psychopharmacol Biol Psychiatry 37:227–236
Jacobson-Pick S, Audet MC, McQuaid RJ, Kalvapalle R, Anisman H (2013) Social agonistic distress in male and female mice: changes of behavior and brain monoamine functioning in relation to acute and chronic challenges. PLoS One 8(4):e60133
Velosky AG, Tucker LB, Fu AH, Liu J, McCabe JT (2017) Cognitive performance of male and female C57BL/6J mice after repetitive concussive brain injuries. Behav Brain Res 324:115–124
Block A, Ahmed MM, Dhanasekazran AR, Tong S, Gardiner K (2015) Sex differences in protein expression in the mouse brain and heir perturbations in a model of Down syndrome. Biol Sex Differ 6:24
Falsafi SK, Dierssen M, Ghafari M, Pollak A, Lubec G (2016) Reduced cortical neurotransmitter receptor complex levels in fetal Down syndrome brain. Amino Acids 48:103–116
Thomazeau A, Lassalle O, Lafrati J et al (2014) Prefrontal deficits in a murine model overexpressing the down syndrome candidate Gene Dyrk1a. J Neurosci 34:1138–1147
De la Fuente M, Hernanz A, Medina S, Guayerbas N, Fernández B, Viveros MP (2003) Characterization of monoaminergic systems in brain regions of prematurely ageing mice. Neurochem Int 43:165–172
Kim DK, Tolliver TJ, Huang SJ et al (2005) Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology 49:798–810
Tsunemi A, Utsuyama M, Seidler BK, Kobayashi S, Hirokawa K (2005) Age-related decline of brain monoamines in mice is reversed to young level by Japanese herbal medicine. Neurochem Res 30:75–81
Chourbaji S, Hellweg R, Brandis D et al (2004) Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and un altered emotional behavior. Brain Res Mol Brain Res 121:28–36
Heller HC, Salehi A, Chuluun B, Das D et al (2014) Nest building is impaired in the Ts65Dn mouse model of Down syndrome and rescued by blocking 5HT2a receptors. Neurobiol Learn Mem 116:162–171
Bianchi P, Ciani E, Guidi S et al (2010) Early pharmacotherapy restores neurogenesis and cognitive performance in the Ts65Dn mouse model for Down syndrome. J Neurosci 30:8769–8779
Guidi S, Stagni F, Bianchi P, Ciani E et al (2013) Early pharmacotherapy with fluoxetine rescues dendritic pathology in the Ts65Dn mouse model of down syndrome. Brain Pathol 23:129–143
Martinez de Lagran M, Bortolozzi A, Millan O et al (2007) Dopaminergic deficiency in mice with reduced levels of the dual-specificity tyrosine-phosphorylated and regulated kinase 1A, Dyrk1A(+/−). Genes Brain Behav 6:569–578
Barallobre MJ, Perier C, Bové J et al (2014) Survival in the developing brain and in a mouse model of Parkinson’s disease. Cell Death Dis 5:e1289
Lockrow J, Boger H, Gerhardt G et al (2011) A noradrenergic lesion exacerbates neurodegeneration in a Down syndrome mouse model. J Alzheimers Dis 23:471–489
Dekker AD, Coppus AM, Vermeiren Y et al (2015) Serum MHPG strongly predicts conversion to Alzheimer’s disease in behaviorally characterized subjects with Down syndrome. J Alzheimers Dis 43:871–891
Yuen EY, Qin L, Wei J et al (2014) Synergistic regulation of glutamatergic transmission by serotonin and norepinephrine reuptake inhibitors in prefrontal cortical neurons. J Biol Chem 289:25177–25185
Wegiel J, Gong CX, Hwang YW (2011) The role of DYRK1A in neurodegenerative diseases. FEBS 278:236–245
Coutadeur S, Benyamine H, Delalonde L, de Oliveira C et al (2015) A novel DYRK1A (dual specificity tyrosine phosphorylation-regulated kinase 1A) inhibitor for the treatment of Alzheimer’s disease: effect on Tau and amyloid pathologies in vitro. J Neurochem 133:440–451
Vermeiren Y, Janssens J, Aerts T, Martin JJ, Sieben A, Van Dam D, De Deyn PP (2016) Brain serotonergic and noradrenergic deficiencies in behavioral variant Fronto temporal dementia compared to early-onset Alzheimer’s disease. J Alzheimers Dis 53:1079–1096
Ledo JH, Beckman D, Ribeiro FC et al (2015) Cross talk between brain innate immunity and serotonin signaling underlies depressive-like behavior induced by Alzheimer’s amyloid-β oligomers in mice. J Neurosci 36:12106–12116
Homberg JR, Molteni R, Calabrese F, Riva MA (2014) The serotonin-BDNF duo: developmental implications for the vulnerability to psychopathology. Neurosci Biobeha Rev 43:35–47
De la Torre R, De Sola S, Pons M et al (2014) Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol Nutri & Food Res 58:278–288
De la Torre R, de Sola S, Hernandez G, Farré M et al (2016) Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down’s syndrome (TESDAD): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol 8:801–810
Stagni F, Giacomini A, Emili M, Trazzi S, Guidi S, Sassi M, Ciani E, Rimondini R et al (2016) Short-and long-term effects of neonatal pharmacotherapy with epigallocatechin-3-gallate on hippocampal development in the Ts65Dn mouse model of Down syndrome. Neuroscience 333:277–301
Kazim SF, Blanchard J, Bianchi R, Iqbal K (2017) Early neurotrophic pharmacotherapy rescues developmental delay and Alzheimer’s-like memory deficits in the Ts65Dn mouse model of Down syndrome. Sci Rep 7:45561
Yin X, Jin N, Shi J, Zhang Y, Wu Y et al (2017) Dyrk1A overexpression leads to increase of 3R-tau expression and cognitive deficits in Ts65Dn Down syndrome mice. Sci Rep 7:45561
Hodgson AB, Randell RK, Mahabir-Jagessar-T K, Lotito S et al (2014) Acute effects of green tea extract intake on exogenous and endogenous metabolites in human plasma. J Agric Food Chem 62:1198–1208
Shimohata A, Ishihara K, Hattori S, Miyamoto H et al (2017) Ts1Cje Down syndrome model mice exhibit environmental stimuli-triggered locomotor hyperactivity and sociability concurrent with increased flux through central dopamine and serotonin metabolism. Exp Neurol 293:1–12
Acknowledgements
We thank the personnel of Institut Jacques Monod animal facility for taking care of the animals for health and control. We thank for funding CNRS, the European Commission (AnEUploidy project LSHG-CT-2006-037627 and the AFRT (Association Française pour la Recherche sur la Trisomie 21) for grants (2013-2015) and financial support for BS, EA, and HM.
Author information
Authors and Affiliations
Contributions
CR, JD, JL designed the study; CR, JD, JL, LCB, performed the HPLC experiments; CR, BS, EA, FD, HM, JL performed the other experiments; CR, EA, JD, JL JMD, LCB analyzed the data; JL and CR wrote the paper. CM, JD, NJ and SL correct the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that the research was performed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors state no actual or potential conflict of interest.
Rights and permissions
About this article
Cite this article
London, J., Rouch, C., Bui, L.C. et al. Overexpression of the DYRK1A Gene (Dual-Specificity Tyrosine Phosphorylation-Regulated Kinase 1A) Induces Alterations of the Serotoninergic and Dopaminergic Processing in Murine Brain Tissues. Mol Neurobiol 55, 3822–3831 (2018). https://doi.org/10.1007/s12035-017-0591-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0591-6