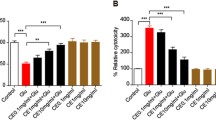
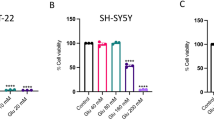
Abstract
Glutamate-induced excitotoxicity is one of the major underlying mechanisms for neurodegenerative diseases. Efforts are being made to treat such conditions with an array of natural compounds that can modulate the release of glutamate or the underlying mechanisms associated with it. Withania somnifera extract has potent pharmacologic activity similar to that of Korean Ginseng tea and is used to treat several neuronal disorders. However, to date, little efforts have been made to evaluate individual constituents of this plant for neurodegenerative disorders. Present study was carried out to investigate withanolide-A, one of the active constituents of Withania somnifera against glutamate-induced excitotoxicity in retinoic acid differentiated Neuro2a neuroblastoma cells. The results indicated that glutamate treatment for 2 h induced death in cells that was significantly attenuated by pre-treatment with MK-801 (specific NMDA receptor antagonist) and different concentrations of withanolide-A. Withanolide-A abated the glutamate-induced influx of intracellular calcium and excessive ROS production significantly. Further on, glutamate treatment resulted in increased levels of pro-apoptotic and decreased levels of anti-apoptotic proteins, and these protein levels were normalized by various doses of withanolide-A. All of these protective effects were partly due to inhibition of MAPK family proteins and activation of PI3K/Akt signaling. Thus, our results suggest that withanolide-A may serve as potential neuroprotective agent.







Similar content being viewed by others
Abbreviations
- NMDA:
-
N-methyl-D-aspartate
- RA:
-
Retinoic acid
- ROS:
-
Reactive oxygen species
- DMSO:
-
Dimethyl sulfoxide
- HRP:
-
Horse reddish peroxidase
- H2DCFDA:
-
2′,7′-dichlorodihydrofluorescein diacetate
- NMDARs:
-
N-methyl-D-aspartate receptor.
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- Glu:
-
Glutamate
- WLA:
-
Withanolide-A
References
Beal MF (1992) Mechanisms of excitotoxicity in neurologic diseases. FASEB J 6(15):3338–3344
Olsen GM, Sonnewald U (2015) Glutamate: Where does it come from and where does it go? Neurochem Int 88:47–52
Hu N-W, Ondrejcak T, Rowan MJ (2012) Glutamate receptors in preclinical research on Alzheimer’s disease: Update on recent advances. Pharmacol Biochem Behav 100(4):855–862
Dar NJ, Bhat JA, Satti NK, Sharma PR, Hamid A, Ahmad M (2016) Withanone, an active constituent from Withania somnifera, affords protection against NMDA-induced excitotoxicity in neuron-like cells. Mol Neurobiol 1–13
Kritis AA, Stamoula EG, Paniskaki KA, Vavilis TD (2015) Researching glutamate–induced cytotoxicity in different cell lines: A comparative/collective analysis/study. Front Cell Neurosci 9:91
Jiang Q, Gu Z, Zhang G, Jing G (2000) Diphosphorylation and involvement of extracellular signal-regulated kinases (ERK1/2) in glutamate-induced apoptotic-like death in cultured rat cortical neurons. Brain Res 857(1):71–77
Pi R, Li W, Lee NT, Chan HH, Pu Y, Chan LN, Sucher NJ, Chang DC et al (2004) Minocycline prevents glutamate-induced apoptosis of cerebellar granule neurons by differential regulation of p38 and Akt pathways. J Neurochem 91(5):1219–1230
Molz S, Dal-Cim T, Budni J, Martín-de-Saavedra M, Egea J, Romero A, del Barrio L, Rodrigues AL et al (2011) Neuroprotective effect of guanosine against glutamate-induced cell death in rat hippocampal slices is mediated by the phosphatidylinositol-3 kinase/Akt/glycogen synthase kinase 3β pathway activation and inducible nitric oxide synthase inhibition. J Neurosci Res 89(9):1400–1408
Shah S, Lee H, Bressan R, Yun D, Kim M (2014) Novel osmotin attenuates glutamate-induced synaptic dysfunction and neurodegeneration via the JNK/PI3K/Akt pathway in postnatal rat brain. Cell Death Dis 5(1):e1026
Endo H, Nito C, Kamada H, Nishi T, Chan PH (2006) Activation of the Akt/GSK3β signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J Cereb Blood Flow Metab 26(12):1479–1489
Kawano T, Morioka M, Yano S, Hamada J-I, Ushio Y, Miyamoto E, Fukunaga K (2002) Decreased akt activity is associated with activation of forkhead transcription factor after transient forebrain ischemia in gerbil hippocampus. J Cereb Blood Flow Metab 22(8):926–934
Thomas GM, Huganir RL (2004) MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 5(3):173–183
Sweatt JD (2004) Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 14(3):311–317
Kim EK, Choi E-J (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta (BBA) - Mol Basis Dis 1802(4):396–405
Wiegler K, Bonny C, Coquoz D, Hirt L (2008) The JNK inhibitor XG-102 protects from ischemic damage with delayed intravenous administration also in the presence of recombinant tissue plasminogen activator. Cerebrovasc Dis 26(4):360–366
Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C (2003) A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med 9(9):1180–1186
Zhou X, Hollern D, Liao J, Andrechek E, Wang H (2013) NMDA receptor-mediated excitotoxicity depends on the coactivation of synaptic and extrasynaptic receptors. Cell Death Dis 4(3):e560
Dar NJ, Hamid A, Ahmad M (2015) Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell Mol Life Sci 72(23):4445–4460
Kuboyama T, Tohda C, Komatsu K (2005) Neuritic regeneration and synaptic reconstruction induced by withanolide a. Br J Pharmacol 144(7):961–971
Baitharu I, Jain V, Deep SN, Hota KB, Hota SK, Prasad D, Ilavazhagan G (2013) Withania somnifera Root extract ameliorates hypobaric hypoxia induced memory impairment in rats. J Ethnopharmacol 145(2):431–441
Baitharu I, Jain V, Deep SN, Shroff S, Sahu JK, Naik PK, Ilavazhagan G (2014) Withanolide a prevents neurodegeneration by modulating hippocampal glutathione biosynthesis during hypoxia. PLoS One 9(10):e105311. doi:10.1371/journal.pone.0105311
Akhoon BA, Pandey S, Tiwari S, Pandey R (2016) Withanolide a offers neuroprotection, ameliorates stress resistance and prolongs the life expectancy of Caenorhabditis elegans. Exp Gerontol 78:47–56
Lone AM, Dar NJ, Hamid A, Shah WA, Ahmad M, Bhat BA (2015) Promise of retinoic acid-triazolyl derivatives in promoting differentiation of neuroblastoma cells. ACS Chem Neurosci 7(1):82–89
Fairman W, Amara S (1999) Functional diversity of excitatory amino acid transporters: Ion channel and transport modes. Am J Physiol Ren Physiol 277(4):F481–F486
Gao S-F, Bao A-M (2011) Corticotropin-releasing hormone, glutamate, and γ-aminobutyric acid in depression. Neuroscientist 17(1):124–144
Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma P (2013) Excitotoxicity: Bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol 698(1):6–18
Sattler R, Charlton MP, Hafner M, Tymianski M (1998) Distinct influx pathways, not calcium load, determine neuronal vulnerability to calcium neurotoxicity. J Neurochem 71(6):2349–2364
Singh B, Saxena A, Chandan B, Gupta D, Bhutani K, Anand K (2001) Adaptogenic activity of a novel, withanolide-free aqueous fraction from the roots of Withania somnifera dun. Phytother Res 15(4):311–318
RajaSankar S, Manivasagam T, Sankar V, Prakash S, Muthusamy R, Krishnamurti A, Surendran S (2009) Withania somnifera Root extract improves catecholamines and physiological abnormalities seen in a Parkinson’s disease model mouse. J Ethnopharmacol 125(3):369–373
Bhattacharya S, Bhattacharya A, Sairam K, Ghosal S (2000) Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: An experimental study. Phytomedicine 7(6):463–469
Saykally JN, Hatic H, Keeley KL, Jain SC, Ravindranath V, Citron BA (2016) Withania somnifera extract protects model neurons from in vitro traumatic injury. Cell Transplant
Yenisetti SC, Manjunath MJ (2015) Neuropharmacological properties of Withania somnifera-Indian Ginseng: An overview on experimental evidence with emphasis on clinical trials and patents. Recent Pat on CNS Drug Discov 10(2):204–215
Ahmad M, Saleem S, Ahmad AS, Ansari MA, Yousuf S, Hoda MN, Islam F (2005) Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced parkinsonism in rats. Hum Exp Toxicol 24(3):137–147
Patil SP, Maki S, Khedkar SA, Rigby AC, Chan C (2010) Withanolide a and asiatic acid modulate multiple targets associated with amyloid-β precursor protein processing and amyloid-β protein clearance. J Nat Prod 73(7):1196–1202
Baitharu I, Jain V, Deep SN, Hota KB, Hota SK, Prasad D, Ilavazhagan G (2013) Withania somnifera Root extract ameliorates hypobaric hypoxia induced memory impairment in rats. J Ethnopharmacol 145(2):431–441. doi:10.1016/j.jep.2012.10.063
Soman S, Anju T, Jayanarayanan S, Antony S, Paulose C (2013) Impaired motor learning attributed to altered AMPA receptor function in the cerebellum of rats with temporal lobe epilepsy: Ameliorating effects of Withania somnifera and withanolide a. Epilepsy Behav 27(3):484–491
Choudhary MI, Nawaz SA, Lodhi MA, Ghayur MN, Jalil S, Riaz N, Yousuf S, Malik A et al (2005) Withanolides, a new class of natural cholinesterase inhibitors with calcium antagonistic properties. Biochem Biophys Res Commun 334(1):276–287
Grunze H, Langosch J, von Loewenich C, Walden J (2000) Modulation of neural cell membrane conductance by the herbal anxiolytic and antiepileptic drug aswal. Neuropsychobiology 42(Suppl. 1):28–32
Gagliardi RJ (2000) Neuroprotection, excitotoxicicity and NMDA antagonists. Arq Neuropsiquiatr 58(2B):583–588
Liu X, Ma C, Xing R, Zhang W, Tian B, Li X, Li Q, Zhang Y (2013) The calmodulin-dependent protein kinase II inhibitor KN-93 protects rat cerebral cortical neurons from N-methyl-D-aspartic acid-induced injury. Neural Regen Res 8(2):111
Nicholls D (2004) Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr Mol Med 4(2):149–177
Reynolds IJ, Hastings TG (1995) Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci 15(5):3318–3327
Lee H-G, Zhu X, Casadesus G, Pallàs M, Camins A, O’Neill MJ, Nakanishi S, Perry G et al (2009) The effect of mGluR2 activation on signal transduction pathways and neuronal cell survival. Brain Res 1249:244–250
Farooqui T, Farooqui AA (2009) Aging: An important factor for the pathogenesis of neurodegenerative diseases. Mech Ageing Dev 130(4):203–215
Qin Z-H, Chen R-W, Wang Y, Nakai M, Chuang D-M, Chase TN (1999) Nuclear factor κB nuclear translocation upregulates c-Myc and p53 expression during NMDA receptor-mediated apoptosis in rat striatum. J Neurosci 19(10):4023–4033
Morrison RS, Kinoshita Y (2000) The role of p53 in neuronal cell death. Cell Death Differ 7(10):868
Li H, Zhu H, Xu C-J, Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94(4):491–501
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94(4):481–490
Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ (2000) Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science 290(5497):1761–1765
König H-G, Rehm M, Gudorf D, Krajewski S, Gross A, Ward MW, Prehn JH (2007) Full length bid is sufficient to induce apoptosis of cultured rat hippocampal neurons. BMC Cell Biol 8(1):7
Ward MW, Rehm M, Duessmann H, Kacmar S, Concannon CG, Prehn JH (2006) Real time single cell analysis of bid cleavage and bid translocation during caspase-dependent and neuronal caspase-independent apoptosis. J Biol Chem 281(9):5837–5844
Simonishvili S, Jain MR, Li H, Levison SW, Wood TL (2013) Identification of Bax-interacting proteins in oligodendrocyte progenitors during glutamate excitotoxicity and perinatal hypoxia–ischemia. ASN neuro 5(5):AN20130027
Das A, McDowell M, Pava MJ, Smith JA, Reiter RJ, Woodward JJ, Varma AK, Ray SK et al (2010) The inhibition of apoptosis by melatonin in VSC4. 1 motoneurons exposed to oxidative stress, glutamate excitotoxicity, or TNF-α toxicity involves membrane melatonin receptors. J Pineal Res 48(2):157–169
Culmsee C, Mattson MP (2005) p53 in neuronal apoptosis. Biochem Biophys Res Commun 331(3):761–777
Feng Z, Jin S, Zupnick A, Hoh J, De Stanchina E, Lowe S, Prives C, Levine A (2006) p53 tumor suppressor protein regulates the levels of huntingtin gene expression. Oncogene 25(1):1–7
Culmsee C, Zhu X, Yu QS, Chan SL, Camandola S, Guo Z, Greig NH, Mattson MP (2001) A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid β-peptide. J Neurochem 77(1):220–228
Duan W, Zhu X, Ladenheim B, Yu QS, Guo Z, Oyler J, Cutler RG, Cadet JL et al (2002) p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann Neurol 52(5):597–606
Lau D, Bading H (2009) Synaptic activity-mediated suppression of p53 and induction of nuclear calcium-regulated neuroprotective genes promote survival through inhibition of mitochondrial permeability transition. J Neurosci 29(14):4420–4429
Becattini B, Culmsee C, Leone M, Zhai D, Zhang X, Crowell KJ, Rega MF, Landshamer S et al (2006) Structure–activity relationships by interligand NOE-based design and synthesis of antiapoptotic compounds targeting bid. Proc Natl Acad Sci 103(33):12602–12606
Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M, Blomgren K, Plesnila N (2005) Apoptosis-inducing factor triggered by poly (ADP-ribose) polymerase and bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci 25(44):10262–10272
Pinton P, Rizzuto R (2006) Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death & Differentiation 13(8):1409–1418
Sánchez-Gómez MV, Alberdi E, Pérez-Navarro E, Alberch J, Matute C (2011) Bax and calpain mediate excitotoxic oligodendrocyte death induced by activation of both AMPA and kainate receptors. J Neurosci 31(8):2996–3006
Sarafian TA, Vartavarian L, Kane DJ, Bredesen DE, Verity MA (1994) Bcl-2 expression decreases methyl mercury-induced free-radical generation and cell killing in a neural cell line. Toxicol Lett 74(2):149–155
Lai TW, Zhang S, Wang YT (2014) Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog Neurobiol 115:157–188
Yang DD, Kuan C-Y, Whitmarsh AJ, Rinócn M, Zheng TS, Davis RJ, Rakic P, Flavell RA (1997) Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 389(6653):865–870
Molz S, Decker H, Dal-Cim T, Cremonez C, Cordova FM, Leal RB, Tasca CI (2008) Glutamate-induced toxicity in hippocampal slices involves apoptotic features and p38MAPK signaling. Neurochem Res 33(1):27–36
Alessandrini A, Namura S, Moskowitz MA, Bonventre JV (1999) MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Natl Acad Sci 96(22):12866–12869
Skaper SD, Facci L, Strijbos PJ (2001) Neuronal protein kinase signaling cascades and excitotoxic cell death. Ann N Y Acad Sci 939(1):11–22
Centeno C, Repici M, Chatton J, Riederer B, Bonny C, Nicod P, Price M, Clarke P et al (2007) Role of the JNK pathway in NMDA-mediated excitotoxicity of cortical neurons. Cell Death & Differentiation 14(2):240–253
Hirt L, Badaut J, Thevenet J, Granziera C, Regli L, Maurer F, Bonny C, Bogousslavsky J (2004) D-JNKI1, a cell-penetrating c-Jun-N-terminal kinase inhibitor, protects against cell death in severe cerebral ischemia. Stroke 35(7):1738–1743
Legos JJ, Erhardt JA, White RF, Lenhard SC, Chandra S, Parsons AA, Tuma RF, Barone FC (2001) SB 239063, a novel p38 inhibitor, attenuates early neuronal injury following ischemia. Brain Res 892(1):70–77
LePage KT, Dickey RW, Gerwick WH, Jester EL, Murray TF (2005) On the use of neuro-2a neuroblastoma cells versus intact neurons in primary culture for neurotoxicity studies. Crit Rev Neurobiol 17(1)
Provost P (2010) Interpretation and applicability of microRNA data to the context of Alzheimer’s and age-related diseases. Aging (Albany NY) 2(3):166–169
Acknowledgements
Dr. Ahmad’s work was supported by Ramalingaswamy Fellowship of Department of Biotechnology and financial assistance (MLP6009) as well as logistic support from Council for Scientific and Industrial Research. Dr. Hamid’s research is supported by Department of Biotechnology (BT/PR/3140/PBD/17/656/2009). Additionally, research in both labs is supported by BSC-0108. Mr. Dar is thankful to University Grants Commission, India for Ph.D. research fellowship. Authors are thankful to director of the institute, Dr. Ram Vishwakarma for facilitating the work. Institutional Publication number of this manuscript is IIIM/2019/2017.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dar, N.J., Satti, N.K., Dutt, P. et al. Attenuation of Glutamate-Induced Excitotoxicity by Withanolide-A in Neuron-Like Cells: Role for PI3K/Akt/MAPK Signaling Pathway. Mol Neurobiol 55, 2725–2739 (2018). https://doi.org/10.1007/s12035-017-0515-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0515-5