Abstract
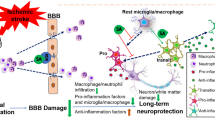
Caspase-1 is an enzyme implicated in neuroinflammation, a critical component of many diseases that affect neuronal degeneration. However, it is unknown whether a caspase-1 inhibitor can modify apoptotic neuronal damage incurred during transient global cerebral ischemia (GCI) and whether intranasal administration of a caspase-1 inhibitor is an effective treatment following GCI. The present study was conducted to examine the potential efficiency of post-ischemic intranasal administration of the caspase-1 inhibitor Boc-D-CMK in a 4-vessel occlusion model of GCI in the rat. Herein, we show that intranasal Boc-D-CMK readily penetrated the central nervous system, subsequently inhibiting caspase-1 activity, decreasing mitochondrial dysfunction, and attenuating caspase-3-dependent apoptotic pathway in ischemia-vulnerable hippocampal CA1 region. Further investigation regarding the mechanisms underlying Boc-D-CMK’s neuroprotective effects revealed marked inhibition of reactive gliosis, as well as reduction of the neuroinflammatory response via inhibition of the downstream pro-inflammatory cytokine production. Intranasal Boc-D-CMK post-treatment also significantly enhanced the numbers of NeuN-positive cells while simultaneously decreasing the numbers of TUNEL-positive and PARP1-positive cells in hippocampal CA1. Correspondingly, behavioral tests showed that deteriorations in spatial learning and memory performance, and long-term recognition memory following GCI were significantly improved in the Boc-D-CMK post-treated animals. In summary, the current study demonstrates that the caspase-1 inhibitor Boc-D-CMK coordinates anti-inflammatory and anti-apoptotic actions to attenuate neuronal death in the hippocampal CA1 region following GCI. Furthermore, our data suggest that pharmacological inhibition of caspase-1 is a promising neuroprotective strategy to target ischemic neuronal injury and functional deficits following transient GCI.










Similar content being viewed by others
References
Mozaffarian D, Benjamin EJ, AS G, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, et al. (2015) Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131(4):e29–322. doi:10.1161/CIR.0000000000000152
Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G (2006) Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab 26(6):821–835. doi:10.1038/sj.jcbfm.9600234
Safar P (1993) Cerebral resuscitation after cardiac arrest: research initiatives and future directions. Ann Emerg Med 22(2 Pt 2):324–349
Zhang QG, Han D, Wang RM, Dong Y, Yang F, Vadlamudi RK, Brann DW (2011) C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-alpha and the critical period hypothesis of estrogen neuroprotection. Proc Natl Acad Sci U S A 108(35):E617–E624. doi:10.1073/pnas.1104391108
Safar P (1986) Cerebral resuscitation after cardiac arrest: a review. Circulation 74(6 Pt 2):IV138–IV153
Harukuni I, Bhardwaj A (2006) Mechanisms of brain injury after global cerebral ischemia. Neurol Clin 24(1):1–21. doi:10.1016/j.ncl.2005.10.004
Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79(4):1431–1568
Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K, Itoyama Y (1995) Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke J Cereb Circ 26(8):1478–1489
Burke DT, Shah MK, Dorvlo AS, Al-Adawi S (2005) Rehabilitation outcomes of cardiac and non-cardiac anoxic brain injury: a single institution experience. Brain Inj 19(9):675–680. doi:10.1080/02699050400024953
Silva BC, de Miranda AS, Rodrigues FG, Silveira AL, Resende GH, Moraes MF, de Oliveira AC, Parreiras PM, et al. (2015) The 5-lipoxygenase (5-LOX) inhibitor zileuton reduces inflammation and infarct size with improvement in neurological outcome following cerebral ischemia. Curr Neurovasc Res 12(4):398–403
Onetti Y, Dantas AP, Perez B, Cugota R, Chamorro A, Planas AM, Vila E, Jimenez-Altayo F (2015) Middle cerebral artery remodeling following transient brain ischemia is linked to early postischemic hyperemia: a target of uric acid treatment. Am J Phys Heart Circ Phys 308(8):H862–H874. doi:10.1152/ajpheart.00001.2015
Shao ZQ, Liu ZJ (2015) Neuroinflammation and neuronal autophagic death were suppressed via rosiglitazone treatment: new evidence on neuroprotection in a rat model of global cerebral ischemia. J Neurol Sci 349(1-2):65–71. doi:10.1016/j.jns.2014.12.027
Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J (1996) Human ICE/CED-3 protease nomenclature. Cell 87(2):171
Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, et al. (1992) A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356(6372):768–774. doi:10.1038/356768a0
Howard AD, Kostura MJ, Thornberry N, Ding GJ, Limjuco G, Weidner J, Salley JP, Hogquist KA, et al. (1991) IL-1-converting enzyme requires aspartic acid residues for processing of the IL-1 beta precursor at two distinct sites and does not cleave 31-kDa IL-1 alpha. J Immunol 147(9):2964–2969
Zhang WH, Wang X, Narayanan M, Zhang Y, Huo C, Reed JC, Friedlander RM (2003) Fundamental role of the Rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal cell death. Proc Natl Acad Sci U S A 100(26):16012–16017. doi:10.1073/pnas.2534856100
Guegan C, Vila M, Teismann P, Chen C, Onteniente B, Li M, Friedlander RM, Przedborski S (2002) Instrumental activation of bid by caspase-1 in a transgenic mouse model of ALS. Mol Cell Neurosci 20(4):553–562
Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, et al. (2000) Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 288(5464):335–339
Willingham SB, Bergstralh DT, O’Connor W, Morrison AC, Taxman DJ, Duncan JA, Barnoy S, Venkatesan MM, et al. (2007) Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2(3):147–159. doi:10.1016/j.chom.2007.07.009
Pasinelli P, Borchelt DR, Houseweart MK, Cleveland DW, Brown RH Jr (1998) Caspase-1 is activated in neural cells and tissue with amyotrophic lateral sclerosis-associated mutations in copper-zinc superoxide dismutase. Proc Natl Acad Sci U S A 95(26):15763–15768
Kozai TD, Li X, Bodily LM, Caparosa EM, Zenonos GA, Carlisle DL, Friedlander RM, Cui XT (2014) Effects of caspase-1 knockout on chronic neural recording quality and longevity: insight into cellular and molecular mechanisms of the reactive tissue response. Biomaterials 35(36):9620–9634. doi:10.1016/j.biomaterials.2014.08.006
Ross J, Brough D, Gibson RM, Loddick SA, Rothwell NJ (2007) A selective, non-peptide caspase-1 inhibitor, VRT-018858, markedly reduces brain damage induced by transient ischemia in the rat. Neuropharmacology 53(5):638–642. doi:10.1016/j.neuropharm.2007.07.015
Rabuffetti M, Sciorati C, Tarozzo G, Clementi E, Manfredi AA, Beltramo M (2000) Inhibition of caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone induces long-lasting neuroprotection in cerebral ischemia through apoptosis reduction and decrease of proinflammatory cytokines. J Neurosci: Off J Soc Neurosci 20(12):4398–4404
Ray AM, Owen DE, Evans ML, Davis JB, Benham CD (2000) Caspase inhibitors are functionally neuroprotective against oxygen glucose deprivation induced CA1 death in rat organotypic hippocampal slices. Brain Res 867(1-2):62–69
Zhang QG, Wang RM, Scott E, Han D, Dong Y, Tu JY, Yang F, Reddy Sareddy G, et al. (2013) Hypersensitivity of the hippocampal CA3 region to stress-induced neurodegeneration and amyloidogenesis in a rat model of surgical menopause. Brain J Neurol 136(Pt 5):1432–1445. doi:10.1093/brain/awt046
Migliore MM, Vyas TK, Campbell RB, Amiji MM, Waszczak BL (2010) Brain delivery of proteins by the intranasal route of administration: a comparison of cationic liposomes versus aqueous solution formulations. J Pharm Sci 99(4):1745–1761. doi:10.1002/jps.21939
Thorne RG, Pronk GJ, Padmanabhan V, Frey WH 2nd (2004) Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 127(2):481–496. doi:10.1016/j.neuroscience.2004.05.029
Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, Vadlamudi RK, Brann DW (2009) Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci: Off J Soc Neurosci 29(44):13823–13836. doi:10.1523/JNEUROSCI.3574-09.2009
Han D, Scott EL, Dong Y, Raz L, Wang R, Zhang Q (2015) Attenuation of mitochondrial and nuclear p38alpha signaling: a novel mechanism of estrogen neuroprotection in cerebral ischemia. Mol Cell Endocrinol 400:21–31. doi:10.1016/j.mce.2014.11.010
Lu Q, Tucker D, Dong Y, Zhao N, Zhang Q (2015) Neuroprotective and functional improvement effects of methylene blue in global cerebral ischemia. Mol Neurobiol. doi:10.1007/s12035-015-9455-0
Gan SD, Patel KR (2013) Enzyme immunoassay and enzyme-linked immunosorbent assay. J Investig Dermatol 133(9):e12. doi:10.1038/jid.2013.287
Ferguson SA, Law CD, Abshire JS (2012) Developmental treatment with bisphenol A causes few alterations on measures of postweaning activity and learning. Neurotoxicol Teratol 34(6):598–606. doi:10.1016/j.ntt.2012.09.006
Barnes CA (1979) Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 93(1):74–104
Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31(1):47–59
Steckler T, Drinkenburg WH, Sahgal A, Aggleton JP (1998) Recognition memory in rats—I. Concepts and classification. Prog Neurobiol 54(3):289–311
Ennaceur A, Aggleton JP (1994) Spontaneous recognition of object configurations in rats: effects of fornix lesions. Exp Brain Res 100(1):85–92
de Lima MN, Laranja DC, Bromberg E, Roesler R, Schroder N (2005) Pre- or post-training administration of the NMDA receptor blocker MK-801 impairs object recognition memory in rats. Behav Brain Res 156(1):139–143. doi:10.1016/j.bbr.2004.05.016
Botton PH, Costa MS, Ardais AP, Mioranzza S, Souza DO, da Rocha JB, Porciuncula LO (2010) Caffeine prevents disruption of memory consolidation in the inhibitory avoidance and novel object recognition tasks by scopolamine in adult mice. Behav Brain Res 214(2):254–259. doi:10.1016/j.bbr.2010.05.034
Gaskin S, Tardif M, Cole E, Piterkin P, Kayello L, Mumby DG (2010) Object familiarization and novel-object preference in rats. Behav Process 83(1):61–71. doi:10.1016/j.beproc.2009.10.003
Kalogeris T, Bao Y, Korthuis RJ (2014) Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol 2:702–714. doi:10.1016/j.redox.2014.05.006
Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Huttemann M (2013) Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol 47(1):9–23. doi:10.1007/s12035-012-8344-z
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140(6):821–832. doi:10.1016/j.cell.2010.01.040
Hentze H, Lin XY, Choi MS, Porter AG (2003) Critical role for cathepsin B in mediating caspase-1-dependent interleukin-18 maturation and caspase-1-independent necrosis triggered by the microbial toxin nigericin. Cell Death Differ 10(9):956–968. doi:10.1038/sj.cdd.4401264
Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, Bholat Y, Vasilevko V, et al. (2013) A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss. J Neurosci: Off J Soc Neurosci 33(15):6245–6256. doi:10.1523/JNEUROSCI.3672-12.2013
Barnes CA, Jung MW, McNaughton BL, Korol DL, Andreasson K, Worley PF (1994) LTP saturation and spatial learning disruption: effects of task variables and saturation levels. J Neurosci: Off J Soc Neurosci 14(10):5793–5806
Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP (2008) The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behav Neurosci 122(1):16–26. doi:10.1037/0735-7044.122.1.16
Broadbent NJ, Squire LR, Clark RE (2004) Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A 101(40):14515–14520. doi:10.1073/pnas.0406344101
Liu XF, Fawcett JR, Hanson LR, Frey WH 2nd (2004) The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J Stroke Cerebrovasc Dis: Off J Natl Stroke Assoc 13(1):16–23. doi:10.1016/j.jstrokecerebrovasdis.2004.01.005
De Rosa R, Garcia AA, Braschi C, Capsoni S, Maffei L, Berardi N, Cattaneo A (2005) Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc Natl Acad Sci U S A 102(10):3811–3816. doi:10.1073/pnas.0500195102
Capsoni S, Giannotta S, Cattaneo A (2002) Nerve growth factor and galantamine ameliorate early signs of neurodegeneration in anti-nerve growth factor mice. Proc Natl Acad Sci U S A 99(19):12432–12437. doi:10.1073/pnas.192442999
Benchoua A, Guegan C, Couriaud C, Hosseini H, Sampaio N, Morin D, Onteniente B (2001) Specific caspase pathways are activated in the two stages of cerebral infarction. J Neurosci: Off J Soc Neurosci 21(18):7127–7134
Stoll G, Kleinschnitz C, Nieswandt B (2010) Combating innate inflammation: a new paradigm for acute treatment of stroke? Ann N Y Acad Sci 1207:149–154. doi:10.1111/j.1749-6632.2010.05730.x
Danton GH, Dietrich WD (2003) Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol 62(2):127–136
Lu P, Kamboj A, Gibson SB, Anderson CM (2014) Poly(ADP-ribose) polymerase-1 causes mitochondrial damage and neuron death mediated by Bnip3. J Neurosci: Off J Soc Neurosci 34(48):15975–15987. doi:10.1523/JNEUROSCI.2499-14.2014
Cohen A, Barankiewicz J (1987) Metabolic consequences of DNA damage: alteration in purine metabolism following poly(ADP ribosyl)ation in human T-lymphoblasts. Arch Biochem Biophys 258(2):498–503
Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, et al. (2006) Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci U S A 103(48):18308–18313. doi:10.1073/pnas.0606526103
Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL (2006) Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci U S A 103(48):18314–18319. doi:10.1073/pnas.0606528103
Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA (1998) Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem 273(49):32608–32613
Freund-Levi Y, Vedin I, Hjorth E, Basun H, Faxen Irving G, Schultzberg M, Eriksdotter M, Palmblad J, et al. (2014) Effects of supplementation with omega-3 fatty acids on oxidative stress and inflammation in patients with Alzheimer’s disease: the OmegAD study. J Alzheimers Dis: JAD 42(3):823–831. doi:10.3233/JAD-132042
Urrutia PJ, Mena NP, Nunez MT (2014) The interplay between iron accumulation, mitochondrial dysfunction, and inflammation during the execution step of neurodegenerative disorders. Front Pharmacol 5:38. doi:10.3389/fphar.2014.00038
Witte ME, Geurts JJ, de Vries HE, van der Valk P, van Horssen J (2010) Mitochondrial dysfunction: a potential link between neuroinflammation and neurodegeneration? Mitochondrion 10(5):411–418. doi:10.1016/j.mito.2010.05.014
Dutta RK, Kathania M, Raje M, Majumdar S (2012) IL-6 inhibits IFN-gamma induced autophagy in Mycobacterium tuberculosis H37Rv infected macrophages. Int J Biochem Cell Biol 44(6):942–954. doi:10.1016/j.biocel.2012.02.021
Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH (2012) Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol 13(3):255–263. doi:10.1038/ni.2215
Jia G, Cheng G, Gangahar DM, Agrawal DK (2006) Insulin-like growth factor-1 and TNF-alpha regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells. Immunol Cell Biol 84(5):448–454. doi:10.1111/j.1440-1711.2006.01454.x
Kirino T (1982) Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239(1):57–69
Pulsinelli WA, Brierley JB, Plum F (1982) Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol 11(5):491–498. doi:10.1002/ana.410110509
Sulzgruber P, Kliegel A, Wandaller C, Uray T, Losert H, Laggner AN, Sterz F, Kliegel M (2015) Survivors of cardiac arrest with good neurological outcome show considerable impairments of memory functioning. Resuscitation 88:120–125. doi:10.1016/j.resuscitation.2014.11.009
Acknowledgment
This study was supported by Research Grant NS086929 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, USA; an American Heart Association Grant-in-Aid 15GRNT25240004; and by the Priority Academic Program Development of Jiangsu Higher Education Institutions and Open project of Key Laboratory of Brain Diseases Bioimformation (Xuzhou Medical University, JSBL1406), and the Jiangsu Government Scholarship Fund (JS2013245).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of interest
The authors declare that there is no conflict of interest in the current study.
Additional information
Ningjun Zhao and Xiaoying Zhuo contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhao, N., Zhuo, X., Lu, Y. et al. Intranasal Delivery of a Caspase-1 Inhibitor in the Treatment of Global Cerebral Ischemia. Mol Neurobiol 54, 4936–4952 (2017). https://doi.org/10.1007/s12035-016-0034-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0034-9