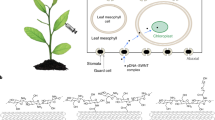
Abstract
Development of efficient, easy, and safe gene delivery methods is of great interest in the field of plant biotechnology. Considering the limitations of the usual transfection methods (such as transgene size and plant type), several new techniques have been tested for replacement. The success of some biological and synthetic nanostructures such as cell-penetrating peptides and carbon nanotubes in transferring macromolecules (proteins and nucleic acids) into mammalian cells provoked us to assess the ability of an engineered chimeric peptide and also arginine functionalized single-walled carbon nanotube in gene delivery to intact tobacco (Nicotiana tabacum var. Virginia) root cells. It was suggested that the engineered peptide with its special cationic and hydrophobic domains and the arginine functionalized single-walled carbon nanotube due to its nano-cylindrical shape can pass plant cell barriers while plasmid DNA (which codes green fluorescent protein) has been condensed on them. The success of gene delivery to tobacco root cells was confirmed by fluorescence microscopy and western blotting analysis.
















Similar content being viewed by others
References
Farkhani, S. M., Valizadeh, A., Karami, H., Mohammadi, S., Sohrabi, N., & Badrzadeh, F. (2014). Cell penetrating peptides: efficient vectors for delivery of nanoparticles, nanocarriers, therapeutic and diagnostic molecules. Peptide, 57, 78–94.
Kim, H., Seo, E. H., Lee, S. H., & Kim, B. J. (2016) The telomerase-derived anticancer peptide vaccine GV1001 as an extracellular heat shock protein-mediated cell-penetrating peptide. International Journal of Molecular Sciences, 17, 2054.
Chugh, A., Amundsen, E., & Eudes, F. (2009). Translocation of cell-penetrating peptides and delivery of their cargoes in triticale microspores. Plant Cell Reports, 28, 801–810.
Unnamalai, N., Kang, B. G., & Lee, W. S. (2004). Cationic oligopeptide-mediated delivery of dsRNA for post-transcriptional gene silencing in plant cells. FEBS Letters, 566, 307–310.
Zonin, E., Moscatiello, R., Miuzzo, M., Cavallarin, N., Dipaolo, M. L., Sandona, D., et al. (2011). Tat-mediated aequorin transduction: An alternative approach for effective calcium measurements in plant cells. Plant and Cell Physiology, 52, 2225–2235.
Rosenbluh, J., Singh, S., Gafni, Y., Graessmann, A., & Loyter, A. (2004). Non-endocytic penetration of core histones into petunia protoplasts and cultured cells: A novel mechanism for the introduction of macromolecules into plant cells. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1664, 230–240.
Lakshmanan, M., Kodama, Y., Yoshizumi, T., Sudesh, K., & Numata, K. (2013). Rapid and efficient gene delivery into plant cells using designed peptide carriers. Biomacromolecules., 14, 10–16.
Numata, K., Ohtani, M., Yoshizumi, T., Demura, T., & Kodama, Y. (2014). Local gene silencing in plants via synthetic dsRNA and carrier peptide. Plant Biotechnology Journal, 12, 1027–1034.
Chuah, J., & Numata, K. (2018). Stimulus-responsive peptide for effective delivery and release of DNA in plants. Biomacromolecules, 19, 1154–1163.
Chuah, J., Yoshizumi, T., Kodama, Y., & Numata, K. (2015). Gene introduction into the mitochondria of Arabidopsis thaliana via peptide-based carriers. Science Reports, 5, 7751.
Ng, K. K., Motoda, Y., Watanabe, S., Othman, A. S., Kigawa, T., Kodama, Y., et al. (2016). Intracellular delivery of proteins via fusion peptides in intact plants. PLoS ONE, 11, 1–19.
Pantarotto, D., Singh, R., McCarthy, D., Erhardt, M., Briand, J. P., Prato, M., et al. (2004). Functionalized carbon nanotubes for plasmid DNA gene delivery. Angewandte Chemie International Edition, 43, 5242–5246.
Liu, Y., Wu, D. C., Zhang, W. D., Jiang, X., He, C. B., Chung, T. S., et al. (2005). Polyethylenimine-grafted multiwalled carbon nanotubes for secure noncovalent immobilization and efficient delivery of DNA. Angewandte Chemie International Edition, 44, 4782–4785.
Foillard, S., Zuber, G., & Doris, E. (2011). Polyethylenimine–carbon nanotube nanohybrids for siRNA-mediated gene silencing at cellular level. Nanoscale, 3, 1461–1464.
Richard, C., Mignet, N., Largeau, C., Escriou, V., Bessodes, M., & Scherman, D. (2009). Functionalization of single- and multi-walled carbon nanotubes with cationic amphiphiles for plasmid DNA complexation and transfection. Nano Research, 2, 638–647.
Khodakovskaya, M., Dervishi, E., Mahmood, M., Xu, Y., Li, Z., Watanabe, F., et al. (2009). Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano, 3, 3221–3227.
Khodakovskaya, M., De Silva, K., Biris, A. S., Dervishi, E., & Villagarcia, H. (2012). Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano, 6, 2128–2135.
Chen, G., Qiu, J., Liu, Y., Jiang, R., Cai, S., Liu, Y., et al. (2015). Carbon nanotubes act as contaminant carriers and translocate within plants. Science Reports, 5, 15682.
Liu, Q., Chen, B., Wang, Q., Shi, X., Xiao, Z., Lin, J., et al. (2009). Carbon nanotubes as molecular transporters for walled plant cells. Nano Letters, 9, 1007–1010.
Burlaka, O. M., Pirco, Ya. V., Yemets, A. L., & Blume, Ya. B. (2015). Plant genetic transformation using carbon nanotubes for DNA delivery. Cytology and Genetics, 49, 3–12.
Majidi, A., Nikkhah, M., Sadeghian, F., & Hosseinkhani, S. (2016). Development of novel recombinant biomimetic chimeric MPG-based peptide as nanocarriers for gene delivery: Imitation of a real cargo. European Journal of Pharmaceutics and Biopharmaceutics, 107, 191–204.
Majidi, A., Nikkhah, M., Sadeghian, F., & Hosseinkhani, S. (2015). Design and bioinformatics analysis of novel biomimetic peptides as nanocarriers for gene transfer. Nanomedicine Journal, 2, 29–38.
Morris, M. C., Vidal, P., Chaloin, L., Heitz, F., & Divita, G. (1997). A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Research, 25, 2730–2736.
Simeoni, F., Morris, M. C., Heitz, F., & Divita, G. (2003). Insight into the mechanism of the peptide-based gene delivery system MPG: Implications for delivery of siRNA into mammalian cells. Nucleic Acids Research, 31, 2717–2724.
Charbgoo, F., Behmanesh, M., & Nikkhah, M. (2015). Enhanced reduction of single-wall carbon nanotube cytotoxicity in vitro: Applying a novel method of arginine functionalization. Biotechnology and Applied Biochemistry, 62, 598–605.
Pompeo, F., & Resasco, D. E. (2002). Water solubilization of single-walled carbon nanotubes by functionalization glucosamine. Nano Letter, 2, 369–373.
Zhang, L., Wang, X. J., Wang, J., Grinberg, N., Krishnamurthy, D., & Senanayake, C. H. (2009). An improved method of amide synthesis using acyl chlorides. Tetrahedron Letters, 50, 2964–2966.
Chiu, W., Niwa, Y., Zeng, W., Hirano, T., Kobayashi, H., & Sheen, J. (1996). Engineered GFP as a vital reporter in plants. Current Biology, 6, 325–330.
Mirzapoor, A., & Ranjbar, B. (2017). Biophysical and electrochemical properties of self-assembled noncovalent SWNT/DNA hybrid and electroactive nanostructure. Physica E: Low-dimensional Systems and Nanostructures, 93, 208–215.
Zhai, Z., Hung, H., & Vatamaniuk, O. K. (2009). Isolation of protoplasts from tissues of 14-day-old seedlings of Arabidopsis thaliana. Journal of Visualized Experiments, 30, 1149.
Wang, W., Vignani, R., Scali, M., & Cresti, M. (2006). A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis, 27, 2782–2786.
Mahmood, T., & Yang, P. (2012). Western blot: Technique, theory and trouble shooting. North American Journal of Medical Sciences, 4, 429–434.
Canine, B. F., & Hatefi, A. (2010). Development of recombinant cationic polymers for gene therapy research. Advanced Drug Delivery Reviews, 62, 1524–1529.
Morris, M. C., Deshayes, S., Heitz, F., & Divita, G. (2008). Cell-penetrating peptides: From molecular mechanisms to therapeutics. Biology of the Cell, 100, 201–217.
Serag, M., Kaji, N., Gaillard, C., Okamoto, Y., Terasaka, K., Jabasini, M., et al. (2011). Trafficking and subcellular localizatio of multiwalled carbon nanotubes in plant cells. ACS Nano, 5, 493–499.
Yaron, P. N., Holt, B. D., Short, P. A., Losche, M., Islam, M. F., & Dahl, K. N. (2011). Single wall carbon nanotubes enter cells by endocytosis and not membrane penetration. Journal of Nanobiotechnology, 9, 45.
Chen, C. P., Chou, J. C., Liu, B. R., Chang, M., & Lee, H. (2007). Transfection and expression of plasmid DNA in plant cells by an arginine-rich intracellular delivery peptide without protoplast preparation. FEBS Letters, 581, 1891–1897.
Chugh, A., & Eudes, F. (2008). Study of uptake of cell penetrating peptides and their cargoes in permeabilized wheat immature embryos. FEBS Journal, 275, 2403–2414.
Zhang, Y., Li, J., Shen, Y., Wang, M., & Li, J. (2004). Poly-l-lysine functionalization of single-walled carbon nanotubes. The Journal of Physical Chemistry B, 108, 15343–15346.
Ghiadi, B., Baniadam, M., Maghrebi, M., & Amiri, A. (2013). Rapid one-pot synthesis of highly-soluble carbon nanotubes functionalized by l-arginine. Russian Journal of Physical Chemistry A, 87, 649–653.
Schaffer, D. V., Fidelman, N. A., Dan, N., & Lauffenburger, D. A. (1999). Vector unpacking as a potential barrie for receptor-mediated polyplex gene delivery. Biotechnology and Bioengineering, 67, 598–606.
Cohen, R. N., Aa, M. A., Macaraeg, N., Lee, A. P., & Szoka, F. J. (2009). Quantification of plasmid DNA copies in the nucleus after lipoplex and polyplex transfection. Journal of Control Release, 135, 166–174.
El-Sayed, A., Futaki, S., & Harashima, H. (2009). Delivery of macromolecules using arginine-rich cell-penetrating peptides: Ways to overcome endosomal entrapment. AAPS Journal, 11, 13–22.
Lundberg, P., El-Andaloussi, S., Sutlu, T., Johansson, H., & Langel, U. (2007). Delivery of short interfering RNA using endosomolytic cell-penetrating peptides. The FASEB Journal, 21, 2664–2671.
Fang, Y., Akula, C., & Altpeter, F. (2002). Agrobacterium-mediated barley (Hordeum vulgare L.) transformation using green fluorescent protein as a visual marker and sequence analysis of the T-DNA: Barely genomic DNA junctions. Journal of Plant Physiology, 159, 1131–1138.
Chang, M., Chou, J. C., & Lee, H. J. (2005). Cellular internalization of fluorescent proteins via arginine-rich intracellular delivery peptide in plant cells. Plant and Cell Physiology, 46, 482–488.
Winkel-shirley, B. (2002). Biosynthesis of flavonoids and effects of stress. Current Opinion in Plant Biology, 5, 218–223.
Petrussa, E., Braidot, E., Zancani, M., Peresson, C., Bertolini, A., Patui, S., et al. (2013). Plant flavonoids—Biosynthesis, transport and involvement in stress responses. International Journal of Molecular Sciences, 14, 14950–14973.
Kasote, D. M., Katyara, S. S., Hegde, M. V., & Bae, H. (2015). Significance of antioxidant potential of plants and its relevance to therapeutic applications. International Journal of Biological Sciences, 11, 982–991.
Talamond, P., Verdeil, J. L., & Conejero, G. (2015). Secondary metabolite localization by autofluorescence in living plant cells. Molecules., 20, 5024–5037.
Carpita, N., Sabularse, D., Montezinos, D., & Delmer, D. P. (1979). Determination of the pore size of the cell walls of living plant cells. Science, 205, 1144–1147.
Meiners, S., Gharyal, P. K., & Schindler, M. (1991). Permeabilization of the plasmalemma and wall of soybean root cells to macromolecules. Planta, 184, 443–447.
Metraux, J. P., & Taiz, L. (1977) Cell wall extension in nitella as influenced by acids and ions. Proceedings of the National Academy of Sciences of the United States of America, 74, 1565–1569.
Cosgrove, D. J. (2000). Loosening of plant cell walls by expansins. Nature., 407, 321–326.
Sadeghian, F., Hosseinkhani, S., Alizadeh, A., & Hatefi, A. (2012). Design, engineering and preparation of a multi-domain fusion vector for gene delivery. International Journal of Pharmaceutics, 427, 393–399.
Acknowledgements
Nanobiotechnology Research Council of Tarbiat Modares University provided financial support of this work. Dr. Asia Majidi performed peptide carrier gene cloning and expression in this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Golestanipour, A., Nikkhah, M., Aalami, A. et al. Gene Delivery to Tobacco Root Cells with Single-Walled Carbon Nanotubes and Cell-Penetrating Fusogenic Peptides. Mol Biotechnol 60, 863–878 (2018). https://doi.org/10.1007/s12033-018-0120-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-018-0120-5