Abstract
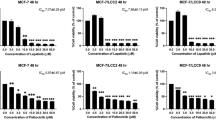
Hormone-dependent breast cancer is the most abundant molecular subtype of the disease. Despite the availability of endocrine treatments, the use of these drugs is limited by their serious adverse reactions and development of acquired resistance often mediated by growth factor receptors. The hepatocyte growth factor receptor, MET, is a receptor tyrosine kinase known for its oncogenic activity and mediating resistance to targeted therapies. Crizotinib is a small-molecule tyrosine kinase inhibitor of MET. In this study, the anticancer effects of combined crizotinib and endocrine drugs were investigated in breast cancer cells in vitro along with the molecular mechanisms associated with these effects. Results showed that crizotinib inhibited growth of MCF7 and T-47D breast cancer cells in a dose-dependent manner with IC50 values of 2.88 μM and 0.93 μM, respectively. Combined treatment of crizotinib and 4-hydroxytamoxifen resulted in synergistic growth inhibition of MCF7 and T-47D cells with combination index values of 0.39 and 0.8, respectively. The combined treatment significantly suppressed migration and colony formation of MCF7 and T-47D cells. Immunofluorescence showed a significant reduction of the expression of the nuclear protein Ki-67 with the combination of crizotinib and 4-hydroxytamoxifen in both cell lines. Western blotting indicated that the combination treatment reduced the levels of active and total MET, estrogen receptor α (ERα), total and active levels of AKT, ERK, c-SRC, NFĸB p65, GSK-3β, and the anti-apoptotic BCL-2 protein. Findings from this study suggest a potential role of MET inhibitors in breast cancer treatment as monotherapy or combination with endocrine drugs.







Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5(10):2929–43.
Badowska-Kozakiewicz AM, Patera J, Sobol M, Przybylski J. The role of oestrogen and progesterone receptors in breast cancer – immunohistochemical evaluation of oestrogen and progesterone receptor expression in invasive breast cancer in women. Contemp Oncol (Pozn). 2015;19(3):220–5.
Munzone E, Colleoni M. Optimal management of luminal breast cancer: how much endocrine therapy is long enough? Ther Adv Med Oncol. 2018;10:1758835918777437.
Patel HK, Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther. 2018;186:1–24.
Zhang Y, Xia M, Jin K, Wang S, Wei H, Fan C, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. 2018;17(1):45.
Demkova L, Kucerova L. Role of the HGF/c-MET tyrosine kinase inhibitors in metastasic melanoma. Mol Cancer. 2018;17(1):26.
Garcia-Vilas JA, Medina MA. Updates on the hepatocyte growth factor/c-Met axis in hepatocellular carcinoma and its therapeutic implications. World J Gastroenterol. 2018;24(33):3695–708.
Zhao X, Qu J, Hui Y, Zhang H, Sun Y, Liu X, et al. Clinicopathological and prognostic significance of c-Met overexpression in breast cancer. Oncotarget. 2017;8(34):56758–67.
Yan S, Jiao X, Zou H, Li K. Prognostic significance of c-Met in breast cancer: a meta-analysis of 6010 cases. Diagn Pathol. 2015;10:62.
de Melo Gagliato D, Jardim DL, Falchook G, Tang C, Zinner R, Wheler JJ, et al. Analysis of MET genetic aberrations in patients with breast cancer at MD Anderson Phase I unit. Clin Breast Cancer. 2014;14(6):468–74.
Sahu A, Prabhash K, Noronha V, Joshi A, Desai S. Crizotinib: a comprehensive review. South Asian J Cancer. 2013;2(2):91–7.
Kazandjian D, Blumenthal GM, Chen HY, He K, Patel M, Justice R, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist. 2014;19(10):e5–11.
Ayoub NM, Al-Shami KM, Alqudah MA, Mhaidat NM. Crizotinib, a MET inhibitor, inhibits growth, migration, and invasion of breast cancer cells in vitro and synergizes with chemotherapeutic agents. Onco Targets Ther. 2017;10:4869–83.
Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005;11(2 Pt 2):865s–70s.
Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13(4):215.
MR RTL, Niles AL, et al. Cell viability assays. In: Sittampalam GS, Coussens NP, Nelson H, et al., editors. Assay guidance manual. Bethesda: Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2004.
Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro cell migration and invasion assays. J Vis Exp. 2014;88
Siragusa M, Dall’Olio S, Fredericia PM, Jensen M, Groesser T. Cell colony counter called CoCoNut. PLoS One. 2018;13(11):e0205823.
Huang L, Cai M, Zhang X, Wang F, Chen L, Xu M, et al. Combinational therapy of crizotinib and afatinib for malignant pleural mesothelioma. Am J Cancer Res. 2017;7(2):203–17.
Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81.
Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–6.
Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298(3):865–72.
Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep. 2015;11(3):1566–72.
Xu Y, Chen M, Liu C, Zhang X, Li W, Cheng H, et al. Association study confirmed three breast Cancer-specific molecular subtype-associated susceptibility loci in Chinese Han women. Oncologist. 2017;22(8):890–4.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52.
Nicholson RI, Johnston SR. Endocrine therapy–current benefits and limitations. Breast Cancer Res Treat. 2005;93(Suppl 1):S3–10.
Klein DJ, Thorn CF, Desta Z, Flockhart DA, Altman RB, Klein TE. PharmGKB summary: tamoxifen pathway, pharmacokinetics. Pharmacogenet Genomics. 2013;23(11):643–7.
Lee CI, Goodwin A, Wilcken N. Fulvestrant for hormone-sensitive metastatic breast cancer. Cochrane Database Syst Rev. 2017;1:CD011093.
Chen R, Guo S, Yang C, Sun L, Zong B, Li K, et al. Although cMYC contributes to tamoxifen resistance, it improves cisplatin sensitivity in ERpositive breast cancer. Int J Oncol. 2020;56(4):932–44.
Raghav K, Bailey AM, Loree JM, Kopetz S, Holla V, Yap TA, et al. Untying the gordion knot of targeting MET in cancer. Cancer Treat Rev. 2018;66:95–103.
Mo HN, Liu P. Targeting MET in cancer therapy. Chronic Dis Transl Med. 2017;3(3):148–53.
Hiscox S, Jordan NJ, Jiang W, Harper M, McClelland R, Smith C, et al. Chronic exposure to fulvestrant promotes overexpression of the c-Met receptor in breast cancer cells: implications for tumour-stroma interactions. Endocr Relat Cancer. 2006;13(4):1085–99.
Basak P, Chatterjee S, Bhat V, Su A, Jin H, Lee-Wing V, et al. Long non-coding RNA H19 acts as an estrogen receptor modulator that is required for endocrine therapy resistance in ER+ breast Cancer cells. Cell Physiol Biochem. 2018;51(4):1518–32.
McClaine RJ, Marshall AM, Wagh PK, Waltz SE. Ron receptor tyrosine kinase activation confers resistance to tamoxifen in breast cancer cell lines. Neoplasia. 2010;12(8):650–8.
Rothenstein JM, Letarte N. Managing treatment-related adverse events associated with Alk inhibitors. Curr Oncol. 2014;21(1):19–26.
Xiang C, Chen J, Fu P. HGF/met signaling in cancer invasion: the impact on cytoskeleton remodeling. Cancers (Basel). 2017;9(5)
Megiorni F, McDowell HP, Camero S, Mannarino O, Ceccarelli S, Paiano M, et al. Crizotinib-induced antitumour activity in human alveolar rhabdomyosarcoma cells is not solely dependent on ALK and MET inhibition. J Exp Clin Cancer Res. 2015;34:112.
Nair A, Chung HC, Sun T, Tyagi S, Dobrolecki LE, Dominguez-Vidana R, et al. Combinatorial inhibition of PTPN12-regulated receptors leads to a broadly effective therapeutic strategy in triple-negative breast cancer. Nat Med. 2018;24(4):505–11.
Nehoff H, Parayath NN, McConnell MJ, Taurin S, Greish K. A combination of tyrosine kinase inhibitors, crizotinib and dasatinib for the treatment of glioblastoma multiforme. Oncotarget. 2015;6(35):37948–64.
Xu W, Kim JW, Jung WJ, Koh Y, Yoon SS. Crizotinib in combination with everolimus synergistically inhibits proliferation of anaplastic lymphoma kinase positive anaplastic large cell lymphoma. Cancer Res Treat. 2018;50(2):599–613.
Zheng X, He K, Zhang L, Yu J. Crizotinib induces PUMA-dependent apoptosis in colon cancer cells. Mol Cancer Ther. 2013;12(5):777–86.
Ariyawutyakorn W, Saichaemchan S, Varella-Garcia M. Understanding and targeting MET signaling in solid tumors - are we there yet? J Cancer. 2016;7(6):633–49.
Chakraborty S, Balan M, Flynn E, Zurakowski D, Choueiri TK, Pal S. Activation of c-Met in cancer cells mediates growth-promoting signals against oxidative stress through Nrf2-HO-1. Oncogenesis. 2019;8(2):7.
Hamedani FS, Cinar M, Mo Z, Cervania MA, Amin HM, Alkan S. Crizotinib (PF-2341066) induces apoptosis due to downregulation of pSTAT3 and BCL-2 family proteins in NPM-ALK(+) anaplastic large cell lymphoma. Leuk Res. 2014;38(4):503–8.
Funding
This work was supported by a grant from the Deanship of Research at Jordan University of Science and Technology (JUST) [grant number 20180279].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ayoub, N.M., Alkhalifa, A.E., Ibrahim, D.R. et al. Combined crizotinib and endocrine drugs inhibit proliferation, migration, and colony formation of breast cancer cells via downregulation of MET and estrogen receptor. Med Oncol 38, 8 (2021). https://doi.org/10.1007/s12032-021-01458-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01458-1