Abstract
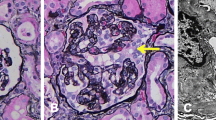
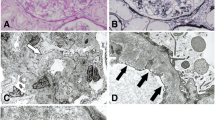
Two patients developed nephrotic syndrome and progressive loss of renal function following treatment with i.v. pamidronate. Renal biopsy revealed collapsing focal segmental glomerulosclerosis (FSGS) in both the patients. In one patient, renal function recovered and the nephrotic syndrome disappeared after discontinuation of pamidronate. The second patient became dialysis dependent despite discontinuation of therapy. Nephrotic syndrome due to collapsing FSGS is a serious complication of treatment with bisphosphonates, especially of i.v. pamidronate. Bisphosphonates may also cause renal insufficiency as a result of tubular toxicity. In order to prevent severe nephrotoxicity clinicians should check urinary protein excretion and renal function regularly in patients who receive long-term treatment with i.v. bisphosphonates. In patients with pre-existing renal impairment (estimated GFR below 30 ml/min), bisphosphonates should be used with caution.


Similar content being viewed by others
References
Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488–93.
Hortobagyi GN, Theriault RL, Lipton A, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol. 1998;16(6):2038–44.
Polascik TJ, Given RW, Metzger C, et al. Open-label trial evaluating the safety and efficacy of zoledronic acid in preventing bone loss in patients with hormone-sensitive prostate cancer and bone metastases. Urology. 2005;66(5):1054–9.
Michaelson MD, Smith MR. Bisphosphonates for treatment and prevention of bone metastases. J Clin Oncol. 2005;23(32):8219–24.
Tanvetyanon T, Stiff PJ. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol. 2006;17(6):897–907.
K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266.
D’Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43(2):368–82.
Schwimmer JA, Markowitz GS, Valeri A, Appel GB. Collapsing glomerulopathy. Semin Nephrol. 2003;23(2):209–18.
Deegens JK, Steenbergen EJ, Borm GF, Wetzels JF. Pathological variants of focal segmental glomerulosclerosis in an adult Dutch population–epidemiology and outcome. Nephrol Dial Transplant. 2008;23(1):186–92.
Markowitz GS, Appel GB, Fine PL, et al. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol. 2001;12(6):1164–72.
Desikan R, Veksler Y, Raza S, et al. Nephrotic proteinuria associated with high-dose pamidronate in multiple myeloma. Br J Haematol. 2002;119(2):496–9.
Kunin M, Kopolovic J, Avigdor A, Holtzman EJ. Collapsing glomerulopathy induced by long-term treatment with standard-dose pamidronate in a myeloma patient. Nephrol Dial Transplant. 2004;19(3):723–6.
Barri YM, Munshi NC, Sukumalchantra S, et al. Podocyte injury associated glomerulopathies induced by pamidronate. Kidney Int. 2004;65(2):634–41.
Shreedhara M, Fenves AZ, Benavides D, Stone MJ. Reversibility of pamidronate-associated glomerulosclerosis. Proc (Bayl Univ Med Cent). 2007;20(3):249–53.
Jotterand V, Moll S, Martin P-Y, Saudan P. Bisphosphonates-induced collapsing focal segmental glomerulosclerosis; two clinical cases and literature review. Nephrologie & Therapeutique. 2009;5:134.
Nagahama M, Sica DA. Pamidronate-induced kidney injury in a patient with metastatic breast cancer. Am J Med Sci. 2009;338(3):225–8.
Markowitz GS, Fine PL, D’Agati VD. Nephrotic syndrome after treatment with pamidronate. Am J Kidney Dis. 2002;39(5):1118–22.
Gokden N, Zangari M, Elici F, Barlogie B, Kumar J. Potential effect of zoledronate therapy in heavy proteinuria. Clin Nephrol. 2007;67(4):263–5.
Pascual J, Torrealba J, Myers J, et al. Collapsing focal segmental glomerulosclerosis in a liver transplant recipient on alendronate. Osteoporos Int. 2007;18(10):1435–8.
Bodmer M, Amico P, Mihatsch MJ, et al. Focal segmental glomerulosclerosis associated with long-term treatment with zoledronate in a myeloma patient. Nephrol Dial Transplant. 2007;22(8):2366–70.
Hiroi-Furuya E, Kameda T, Hiura K, et al. Etidronate (EHDP) inhibits osteoclastic-bone resorption, promotes apoptosis and disrupts actin rings in isolate-mature osteoclasts. Calcif Tissue Int. 1999;64(3):219–23.
Sauter M, Julg B, Porubsky S, et al. Nephrotic-range proteinuria following pamidronate therapy in a patient with metastatic breast cancer: mitochondrial toxicity as a pathogenetic concept? Am J Kidney Dis. 2006;47(6):1075–80.
Markowitz GS, Fine PL, Stack JI, et al. Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney Int. 2003;64(1):281–9.
Smetana S, Michlin A, Rosenman E, Biro A, Boaz M, Katzir Z. Pamidronate-induced nephrotoxic tubular necrosis–a case report. Clin Nephrol. 2004;61(1):63–7.
Buysschaert M, Cosyns JP, Barreto L, Jadoul M. Pamidronate-induced tubulointerstitial nephritis with Fanconi syndrome in a patient with primary hyperparathyroidism. Nephrol Dial Transplant. 2003;18(4):826–9.
Janssen van DK, Neyns B, Van der NP, Verbeelen D. Pamidronate-related nephrotoxicity (tubulointerstitial nephritis) in a patient with osteolytic bone metastases. Nephron. 2001;89(4):467–8.
Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735–44.
McDermott RS, Kloth DD, Wang H, Hudes GR, Langer CJ. Impact of zoledronic acid on renal function in patients with cancer: Clinical significance and development of a predictive model. J Support Oncol. 2006;4(10):524–9.
Diel IJ, Weide R, Köppler H, et al. Risk of renal impairment after treatment with ibandronate versus zoledronic acid: a retrospective medical record review. Support Care Cancer. 2009;17:719–25.
Bergner R, Diel IJ, Henrich D, Hoffman M, Uppenkamp M. Differences in nephrotoxicity of intravenous bisphophonates for the treatment of malignancy related bone disease. Onkologie. 2006;29:534–40.
Body JJ, Pfister T, Bauss F. Preclinical perspectives on bisphosphonate renal safety. Oncologist. 2005;19:3–7.
American Society of Clinical Oncology. Clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25(17):2464–72.
Miller PD, Roux C, Boonen S, Barton IP, Dunlap LE, Burgio DE. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res. 2005;20(12):2105–15.
Acknowledgments
The authors would like to thank H. Dijkman and H. Steenbergen, department of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands, for providing the figures of the kidney biopsies. The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ten Dam, M.A.G.J., Hilbrands, L.B. & Wetzels, J.F.M. Nephrotic syndrome induced by pamidronate. Med Oncol 28, 1196–1200 (2011). https://doi.org/10.1007/s12032-010-9628-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9628-7