Abstract
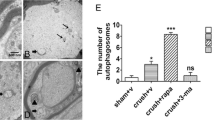
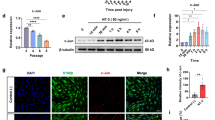
The functional outcome after peripheral nerve repair is often unpredictable for many reasons, e.g., the severity of neuronal death and scarring. Axonal degeneration significantly affects outcomes. Post-injury axonal degeneration in peripheral nerves is accompanied by myelin degradation initiated by Schwann cells (SCs), which activate autophagy, a ubiquitous cytoprotective process essential for degrading and recycling cellular constituents. Scar formation occurs concomitantly with nerve insult and axonal degeneration. The association between SC autophagy and the mechanisms of nerve scar formation is still unknown. A rat model of peripheral nerve lesions induced by sciatic nerve transection injuries was used to examine the function of autophagy in fibrosis reduction during the early phase of nerve repair. Rats were treated with rapamycin (autophagy inducer) or 3-methyladenine (autophagy inhibitor). One week after the nerve damage, fibrosis was potently inhibited in rapamycin-treated rats and, based on gait analysis, yielded a better functional outcome. Immunohistochemistry showed that the autophagic activity of SCs and the accumulation of neurofilaments were upregulated in rapamycin-treated rats. A deficiency of SC autophagic activity might be an early event in nerve scar formation, and modulating autophagy might be a powerful pharmacological approach for improving functional outcomes.






Similar content being viewed by others
References
Abe N, Borson SH, Cavalli V et al (2010) Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. J Biol Chem 285(36):28034–28043
Adanali G, Verdi M, Tuncel A, Erdogan B, Kargi E (2003) Effects of hyaluronic acid-carboxymethylcellulose membrane on extraneural adhesion formation and peripheral nerve regeneration. J Reconstr Microsurg 19:29–36
Bain JR, Mackinnon SE, Hunter RT (1989) Functional evaluation of complete sciatic peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg 83:129–138
Bora FJ (1967) Peripheral nerve repair in cats. The fascicular stitch. J Bone Joint Surg [Am] 49:659–666
Bucko CD, Joynt RL, Grabb WC (1981) Peripheral nerve regeneration in primates during D-penicillamine-induced lathyrism. Plast Reconstr Surg 67:23–30
Caccamo A, Majumder S, Richardson A, Strong R, Oddo S (2010) Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and tau: effects on cognitive impairments. J Biol Chem 285:13107–13120
Chong ZZ, Shang YC, Wang S, Maiese K (2012) Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol 99:128–148
Chu CT (2006) Autophagic stress in neuronal injury and disease. J Neuropathol Exp Neurol 65:423–432
Devor M (2001) Neuropathic pain: what do we do with all these theories? Acta Anaesthesiol Scand 45:1121–1127
Devor M, Seltzer Z (1999) Pathophysiology of damaged nerves in relation to chronic pain. In: Wall PD, Melzack R (eds) Textbook of pain. Churchill Livingstone, London, pp 129–164
Eather TF, Pollock M, Myers DB (1986) Proximal and distal changes in collagen content of peripheral nerve that follow transection and crush lesions. Exp Neurol 92:299–310
Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M, Palomo-Irigoyen M, Varela-Rey M, Griffith M, Hantke J, Macias-Camara N, Azkargorta M, Aurrekoetxea I, de Juan VG, Jefferies HBJ, Aspichueta P, Elortza F, Aransay AM, Martínez-Chantar ML, Baas F, Mato JM, Mirsky R, Woodhoo A, Jessen KR (2015) Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol 210(1):153–168
Gorgulu A, Uzal C, Doganay L, Imer M, Eliuz K, Cobanoglu S (2003) The effect of low-dose external beam radiation on extraneural scarring after peripheral nerve surgery in rats. Neurosurgery 53:1389–1395
Graham W, Patakey P, Calabretta A, Munger B, Buda M (1973) Enhancement of peripheral nerve regeneration with triamcinolone after neurorrhaphy. Surg Forum 24:457–461
Hsiao-Yu L, Tsung-Hsun H, Jia-Jin JC et al (2012) Quantitative video-based gait pattern analysis for hemiparkinsonian rats. Med Biol Eng Comput 50:937–946
Jen-I L, Meng-Yi C, Ming-Long Y et al (2012) Video-based gait analysis for functional evaluation of healing Achilles tendon in rats. Ann Biomed Eng 40(12):2532–2540
Kim SG, Buel GR, Blenis J (2013) Nutrient regulation of the mTOR complex 1 signaling pathway. Mol Cells 35:463–473
Lane JM, Bora FW Jr, Pleasure D (1978) Neuroma scar formation in rats following peripheral nerve transection. J Bone Joint Surg Am 60:197–203
Larsen KE, Sulzer D (2002) Autophagy in neurons: a review. Histol Histopathol 17:897–908
Lundborg G, Danielsen N (1991) Injury, degeneration and regeneration. In: Gelberman RH (ed) Operative nerve repair and reconstruction, vol 1. JB Lippincott, Philadelphia, pp 109–131
Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA (2010) Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci 30:1166–1175
Marinelli S, Pavone F et al (2011) Schwann cell autophagy counteracts the onset and chronification. Pain 155:93–107
Mathur A, Merrell JC, Russell RC, Zook EG (1983) A scanning electron microscopy evaluation of peripheral nerve regeneration. Scan Electron Microsc 1983(Pt 2):975–981
McCallion RL, Ferguson MW (1996) Fetal wound healing and the development of antiscarring therapies for adult wound healing. In: Clark RA (ed) The molecular and cell biology of wound repair, 2nd edn. Plenum Press, New York, pp 561–599
Nachemson AK, Lundborg G, Myrhage R, Rank F (1985) Nerve regeneration after pharmacological suppression of the scar reaction at the suture site. An experimental study on the effect of estrogen-progesterone, methylprednisolone-acetate and cis-hydroxyproline in rat sciatic nerve. Scand J Plast Reconstr Surg 19:255–260
Ozgenel GY (2003) Effects of hyaluronic acid on peripheral nerve scarring and regeneration in rats. Microsurgery 23:575–581
Ozgenel GY, Filiz G (2003) Effects of human amniotic fluid on peripheral nerve scarring and regeneration in rats. J Neurosurg 98:371–377
Ozgenel GY, Filiz G (2004) Combined application of human amniotic membrane wrapping and hyaluronic acid injection in epineurectomized rat sciatic nerve. J Reconstr Microsurg 20:153–157
Peterson J, Russell L, Andrus K, Mackinnon M, Silver J, Kliot M (1996) Reduction of extraneural scarring by ADCON-T/N after surgical intervention. Neurosurgery 38:976–983
Rangaraju S, Verrier JD, Madorsky I, Nicks J, Dunn WA Jr, Notterpek L (2010) Rapamycin activates autophagy and improves myelination in explant cultures from neuropathic mice. J Neurosci 30:11388–11397
Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36:585–595
Robinson PP, Loescher AR, Smith KG (2000) A prospective, quantitative study on the clinical outcome of lingual nerve repair. Br J Oral Maxillofacial Surg 38:255–263
Rubinsztein DC, Difiglia M, Heintz N et al (2005) Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy 1:11–22
Rydevik M, Bergstrom F, Mitts C, Danielson N (2002) Locally applied collagenase and regeneration of transected and repaired rat sciatic nerves. Scand J Plast Reconstr Surg Hand Surg 36:193–196
Sunderland S (1978) Nerves and nerve injuries, 2nd edn. Churchill Livingstone, London, pp 188–200
Sunderland S (1987) Nerves and nerve injuries. Churchill Livingstone, New York, pp 173–176
Sunderland S, Bradley KC (1959) Endoneurial tube shrinkage in the distal segment of a severed nerve. J Comp Neurol 93:411–420
Wehling P, Pak M, Cleveland S, Nieper R (1992) The influence of bacterial collagenase on regeneration of severed rat sciatic nerves. Acta Neurochir 119:121–127
Yates JM, Smith KG, Robinson PP (2000) Ectopic neural activity from myelinated afferent fibres in the lingual nerve of the ferret following three types of injury. Brain Res 874:37–47
Yates JM, Smith KG, Robinson PP (2004) The effect of triamcinolone hexacetonide on the spontaneous and mechanically-induced ectopic discharge following lingual nerve injury in the ferret. Pain 111:261–269
Zou T, Ling C, Xiao Y, Tao X, Ma D, Chen ZL, Strickland S, Song H (2006) Exogenous tissue plasminogen activator enhances peripheral nerve regeneration and functional recovery after injury in mice. J Neuropath Exp Neurol 65:78–86
Acknowledgements
We are grateful to Kuen-Jer Tsai, PhD, Ms. I-Wen Shene, Ms. Shu Hsien Shih, and Ms. Ying-Chiu Lin for their excellent assistance. None of the authors has a commercial interest relevant to the manuscript.
Funding
This study was supported by grant NSC106-2314-B-006-004 from the National Science Council, Taiwan, grant EDAHP 106004 from the E-Da Hospital, Taiwan.
Author information
Authors and Affiliations
Contributions
Po-Yen Ko and Cheng-Chang Yang contributed equally to this work. Po-Yen Ko and Cheng-Chang Yang carried out the study design, acquiring and analyzing the data, and drafting of the articles; Yao-Lung Kuo, Tai-I Hsu participated in the critical revision of the article for important intellectual content, and technical or logical support; Fong-Chin Su, Yuan-Kun Tu, and I-Ming Jou participated in the final approval of the article provision of the study, obtaining of funding, provision of study materials, and critical revision of the article for important intellectual content. All authors have read and approved the final submitted manuscript.
Corresponding authors
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ko, PY., Yang, CC., Kuo, YL. et al. Schwann-Cell Autophagy, Functional Recovery, and Scar Reduction After Peripheral Nerve Repair. J Mol Neurosci 64, 601–610 (2018). https://doi.org/10.1007/s12031-018-1056-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1056-8