Abstract
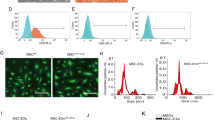
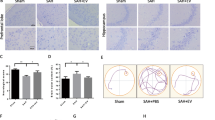
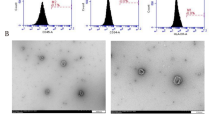
Intracerebral hemorrhage (ICH) has poor outcomes due to high mortality and morbidity, but until now, the effective treatments remain limited. MicroRNAs (miRNAs) are vital regulators of gene expression and demonstrated to be linked to the pathogenesis of various central nervous system (CNS) diseases. Exosomes are considered as cell-to-cell communication vectors and secreted largely by mesenchymal stromal cells (MSCs). The present study investigated the role of miR-133b delivered by exosomes secreted from MSCs to brain tissues in rats after ICH. An autologous arterial blood ICH model in adult male Sprague–Dawley (SD) rats was used in this study. At 72 h after transfection with miR-133b mimics in MSCs, miR-133b-modified MSC-derived exosomes were collected from medium of MSCs and then injected to rats via tail vein. The levels of miR-133b in secreted exosomes and brain tissues of rats in various groups and the levels of RhoA, phosphorylations of extracellular signal regulating kinase (ERK1/2), and cAMP response element-binding protein (CREB) were detected by real-time PCR and western blot analysis, respectively. The effects of miR-133b on neuronal apoptosis and degeneration were respectively evaluated by TUNEL and fluoro-jade B staining. The miR-133b levels were reduced in brain tissues of rats at 24 h and peaked at 72 h after ICH. At 24 h after miR-133b-modified exosome administration, the level of miR-133b was significantly increased, while the apoptotic and neurodegenerative neurons were obviously reduced in brain tissues after ICH. The results of western blot analysis showed that miR-133b modified exosomes treatment remarkably suppressed RhoA expression and activated ERK1/2/CREB in brain tissues after ICH. Collectively, our investigation suggested that exosomes derived from miR-133b modified MSCs exhibited neuroprotective role for anti-apoptotic effect of miR-133b mediating RhoA and ERK1/2/CREB in rats after ICH.





Similar content being viewed by others
Abbreviations
- ICH:
-
Intracerebral hemorrhage
- CNS:
-
Central nervous system
- MSCs:
-
Mesenchymal stromal cells
- ERK1/2:
-
Extracellular signal regulating kinase
- CREB:
-
cAMP response element-binding protein
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
- FJB:
-
Fluoro-jade B
- SBI:
-
Secondary brain injury
- ROS:
-
Reactive oxygen species
- SCI:
-
Spinal cord injury
- ADSCs:
-
Adipose-derived stem cells
References
Akcakaya P, Ekelund S, Kolosenko I, Caramuta S, Ozata DM, Xie H, Lindforss U, Olivecrona H, Lui WO (2011) miR-185 and miR-133b deregulation is associated with overall survival and metastasis in colorectal cancer. Int J Oncol 39:311–318
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345
Aronowski J, Zhao X (2011) Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 42:1781–1786
Bai Y, Wang L, Sun L, Ye P, Hui R (2011) Circulating microRNA-26a: potential predictors and therapeutic targets for non-hypertensive intracerebral hemorrhage. Med Hypotheses 77:488–490
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Bhatnagar S, Schorey JS (2007) Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem 282:25779–25789
Bijian K, Takano T, Papillon J, Le Berre L, Michaud JL, Kennedy CR, Cybulsky AV (2005) Actin cytoskeleton regulates extracellular matrix-dependent survival signals in glomerular epithelial cells. Am J Physiol Renal Physiol 289:F1313–F1323
Dang B, Li H, Xu X, Shen H, Wang Y, Gao A, He W, Wang Z, Chen G (2015) Cyclophilin A/cluster of differentiation 147 interactions participate in early brain injury after subarachnoid hemorrhage in rats. Crit Care Med 43:e369–e381
Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420:629–635
Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G (2002) Behavioral tests after intracerebral hemorrhage in the rat. Stroke 33:2478–2484
Katakowski M, Buller B, Wang X, Rogers T, Chopp M (2010) Functional microRNA is transferred between glioma cells. Cancer Res 70:8259–8263
Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, Shu W, Jiang F, Chopp M (2013) Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett 335:201–204
Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A (2007) A microRNA feedback circuit in midbrain dopamine neurons. Science 317:1220–1224
Li Z, Fang F, Wang Y, Wang L (2016) Resveratrol protects CA1 neurons against focal cerebral ischemic reperfusion-induced damage via the ERK-CREB signaling pathway in rats. Pharmacol Biochem Behav 146-147:21–27
Liu X, Nie S, Huang D, Xie M (2015) Mitogen-activated protein kinase and Akt pathways are involved in 4-n-nonyphenol induced apoptosis in mouse Sertoli TM4 cells. Environ Toxicol Pharmacol 39:815–824
Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35:605–623
Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, Liu Y (2015) Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol 8:122
Lu XC, Zheng JY, Tang LJ, Huang BS, Li K, Tao Y, Yu W, Zhu RL, Li S, Li LX (2015) MiR-133b promotes neurite outgrowth by targeting RhoA expression. Cell Physiol Biochem 35:246–258
Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan JQ, Henshall DC, Simon RP (2005) CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab 25:234–246
Okano H, Yamanaka S (2014) iPS cell technologies: significance and applications to CNS regeneration and disease. Mol Brain 7:22
Otero-Ortega L, Gomez de Frutos MC, Laso-Garcia F, Rodriguez-Frutos B, Medina-Gutierrez E, Lopez JA, Vazquez J, Diez-Tejedor E, Gutierrez-Fernandez M (2017) Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J Cereb Blood Flow Metab. 271678X17708917
Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM (2010) Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A 107:6328–6333
Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF (2001) Spontaneous intracerebral hemorrhage. N Engl J Med 344:1450–1460
Sanchez-Simon FM, Zhang XX, Loh HH, Law PY, Rodriguez RE (2010) Morphine regulates dopaminergic neuron differentiation via miR-133b. Mol Pharmacol 78:935–942
Schaffler A, Buchler C (2007) Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells 25:818–827
Smalheiser NR (2007) Exosomal transfer of proteins and RNAs at synapses in the nervous system. Biol Direct 2:35
Trams EG, Lauter CJ, Salem N Jr, Heine U (1981) Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 645:63–70
Tsai PC, Liao YC, Wang YS, Lin HF, Lin RT, Juo SH (2013) Serum microRNA-21 and microRNA-221 as potential biomarkers for cerebrovascular disease. J Vasc Res 50:346–354
Wang Z, Chen Z, Yang J, Yang Z, Yin J, Zuo G, Duan X, Shen H, Li H, Chen G (2017) Identification of two phosphorylation sites essential for annexin A1 in blood-brain barrier protection after experimental intracerebral hemorrhage in rats. J Cereb Blood Flow Metab 37:2509–2525
Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30:1556–1564
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M (2013a) Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 33:1711–1715
Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M (2013b) MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 31:2737–2746
Xin H, Wang F, Li Y, Lu QE, Cheung WL, Zhang Y, Zhang ZG, Chopp M (2017) Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microRNA 133b-overexpressing multipotent mesenchymal stromal cells. Cell Transplant 26:243–257
Yang Z, Zhong L, Xian R, Yuan B (2015) MicroRNA-223 regulates inflammation and brain injury via feedback to NLRP3 inflammasome after intracerebral hemorrhage. Mol Immunol 65:267–276
Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK (2013) Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev 65:336–341
Zeng Z, Leng T, Feng X, Sun H, Inoue K, Zhu L, Xiong ZG (2015) Silencing TRPM7 in mouse cortical astrocytes impairs cell proliferation and migration via ERK and JNK signaling pathways. PLoS One 10:e0119912
Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y (2015) Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg 122:856–867
Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW (2014) Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol 115:25–44
Zhu Y, Wang JL, He ZY, Jin F, Tang L (2015) Association of altered serum microRNAs with perihematomal edema after acute intracerebral hemorrhage. PLoS One 10:e0133783
Funding
This work was supported by National Natural Science Foundation of China (No. 81501005), grants from the China Postdoctoral Science Foundation (Nos. 2016M591912, 2015M580465), and grants from Jiangsu Planned Projects for Postdoctoral Research Funds (No.1501064A).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal experiments were approved by the Ethics Committee of the First Affiliated Hospital of Soochow University and were performed in accordance with the guidelines of the National Institutes of Health on the care and use of animals.
Conflict of Interest Statement
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Shen, H., Yao, X., Li, H. et al. Role of Exosomes Derived from miR-133b Modified MSCs in an Experimental Rat Model of Intracerebral Hemorrhage. J Mol Neurosci 64, 421–430 (2018). https://doi.org/10.1007/s12031-018-1041-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1041-2