Abstract
This study aimed at obtaining an in-depth mapping of expressional changes of the cerebral microvasculature after transient global cerebral ischemia (GCI) and the impact on these GCI-induced expressional changes of post-GCI treatment with a mitogen-activated protein kinase kinase (MEK1/2) inhibitor. GCI was induced in male Wistar rats followed by treatment with either vehicle or the MEK1/2 inhibitor U0126 every 12 h post-GCI. Seventy-two hours after GCI or sham surgery, the cerebral microvasculature was isolated and the protein content analysed with state-of-the-art mass spectrometry. The proteomic profile of the isolated cerebral microvasculature 72 h after GCI (compared to sham) indicated that the main expressional changes could be divided into nine categories: (1) cellular respiration, (2) remodelling of the extracellular matrix, (3) decreased contractile phenotype, (4) clathrin-mediated endocytosis, (5) ribosomal activity, (6) expression of chromatin structure-related proteins, (7) altered synaptic activity, (8) altered G-protein signalling and (9) instability of the membrane potential. Treatment with U0126 partly normalized the expression of one or more of the proteins in all nine categories. Flow cytometry confirmed key findings from the proteome such as upregulation of the extracellular proteins lamininβ2 and nidogen2 (p < 0.05) after GCI. These results provide valuable molecular insight into the broad and complex expressional changes in the cerebral microvasculature after GCI and the effect of early MEK1/2 inhibitor treatment on these changes.



Similar content being viewed by others
References
Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468:232–243
Baeten KM, Akassoglou K (2011) Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol 71:1018–1039
Bradbury MW (1993) The blood-brain barrier. Exp Physiol 78:453–472
Dascal N (2001) Ion-channel regulation by G proteins. Trends Endocrinol Metab 12:391–398
del Zoppo GJ (2009) Relationship of neurovascular elements to neuron injury during ischemia. Cerebrovasc Dis 27(Suppl 1):65–76
Dienel GA (2014) Lactate shuttling and lactate use as fuel after traumatic brain injury: metabolic considerations. J Cereb Blood Flow Metab 34:1736–1748
Edvinsson L, Krause DN (2002) Cerebral blood flow and metabolism. Lippincott Williams and Wilkins
Edvinsson LI, Povlsen GK (2011) Vascular plasticity in cerebrovascular disorders. J Cereb Blood Flow Metab 31:1554–1571
Engholm-Keller K, Birck P, Storling J, Pociot F, Mandrup-Poulsen T, Larsen MR (2012) TiSH—a robust and sensitive global phosphoproteomics strategy employing a combination of TiO2, SIMAC, and HILIC. J Proteome 75:5749–5761
Findeisen HM, Kahles FK, Bruemmer D (2013) Epigenetic regulation of vascular smooth muscle cell function in atherosclerosis. Curr Atheroscler Rep 15:319.
Haqqani AS, Nesic M, Preston E, Baumann E, Kelly J, Stanimirovic D (2005) Characterization of vascular protein expression patterns in cerebral ischemia/reperfusion using laser capture microdissection and ICAT-nanoLC-MS/MS. FASEB J 19:1809–1821
Ho MS, Bose K, Mokkapati S, Nischt R, Smyth N (2008) Nidogens—extracellular matrix linker molecules. Microsc Res Tech 71:387–395
Johansson S, Povlsen GK, Edvinsson L (2012) Expressional changes in cerebrovascular receptors after experimental transient forebrain ischemia. PLoS One 7:e41852
Johansson SE, Larsen SS, Povlsen GK, Edvinsson L (2014) Early MEK1/2 inhibition after global cerebral ischemia in rats reduces brain damage and improves outcome by preventing delayed vasoconstrictor receptor upregulation. PLoS One 9:e92417
Johansson SE, Andersen XE, Hansen RH, Povlsen GK, Edvinsson L (2015) Cerebrovascular endothelin-1 hyper-reactivity is associated with transient receptor potential canonical channels 1 and 6 activation and delayed cerebral hypoperfusion after forebrain ischaemia in rats. Acta Physiol (Oxf) 214:376–389
Liu R, Leslie KL, Martin KA (2015) Epigenetic regulation of smooth muscle cell plasticity. Biochim Biophys Acta 1849:448–453
Martinez-Lemus LA, Hill MA, Meininger GA (2009a) The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 24:45–57
Martinez-Lemus LA, Hill MA, Meininger GA (2009b) The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 24:45–57
McMahon HT, Boucrot E (2011) Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12:517–533
Mokkapati S, Bechtel M, Reibetanz M, Miosge N, Nischt R (2012) Absence of the basement membrane component nidogen 2, but not of nidogen 1, results in increased lung metastasis in mice. J Histochem Cytochem 60:280–289
Navone SE, Marfia G, Invernici G, Cristini S, Nava S, Balbi S, Sangiorgi S, Ciusani E, Bosutti A, Alessandri G, Slevin M, Parati EA (2013) Isolation and expansion of human and mouse brain microvascular endothelial cells. Nat Protoc 8:1680–1693
Neumann JT, Cohan CH, Dave KR, Wright CB, Perez-Pinzon MA (2013) Global cerebral ischemia: synaptic and cognitive dysfunction. Curr Drug Targets 14:20–35
Pardridge WM, Yang J, Eisenberg J, Tourtellotte WW (1987) Isolation of intact capillaries and capillary plasma membranes from frozen human brain. J Neurosci Res 18:352–357
Petito CK, Feldmann E, Pulsinelli WA, Plum F (1987) Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology 37:1281–1286
Pulsinelli WA, Brierley JB, Plum F (1982) Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol 11:491–498
Rocchiccioli S, Ucciferri N, Comelli L, Trivella MG, Citti L, Cecchettini A (2012) Proteomics changes in adhesion molecules: a driving force for vascular smooth muscle cell phenotypic switch. Mol BioSyst 8:1052–1059
Shimizu-Hirota R, Sasamura H, Kuroda M, Kobayashi E, Hayashi M, Saruta T (2004) Extracellular matrix glycoprotein biglycan enhances vascular smooth muscle cell proliferation and migration. Circ Res 94:1067–1074
Smith ML, Bendek G, Dahlgren N, Rosen I, Wieloch T, Siesjo BK (1984) Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model. Acta Neurol Scand 69:385–401
Steen H, Mann M (2004) The ABC’s (and XYZ’s) of peptide sequencing. Nat Rev Mol Cell Biol 5:699–711
Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C (2015) STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43:D447–D452
van Beijnum JR, Rousch M, Castermans K, van der Linden E, Griffioen AW (2008) Isolation of endothelial cells from fresh tissues. Nat Protoc 3:1085–1091
Wisniewski HM, Pluta R, Lossinsky AS, Mossakowski MJ (1995) Ultrastructural studies of cerebral vascular spasm after cardiac arrest-related global cerebral ischemia in rats. Acta Neuropathol 90:432–440
Yang GY, Chen SF, Kinouchi H, Chan PH, Weinstein PR (1992) Edema, cation content, and ATPase activity after middle cerebral artery occlusion in rats. Stroke 23:1331–1336
Zeng YS, Xu ZC (2000) Co-existence of necrosis and apoptosis in rat hippocampus following transient forebrain ischemia. Neurosci Res 37:113–125
Zhu Z, Boobis AR, Edwards RJ (2008) Identification of estrogen-responsive proteins in MCF-7 human breast cancer cells using label-free quantitative proteomics. Proteomics 8:1987–2005
Acknowledgements
We kindly acknowledge the Lundbeck Foundation for financial support (LE—Grant of Excellence and MRL—Junior Group Leader Fellowship).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
S1 Fig
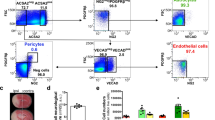
Experimental characteristics of flow cytometry experiments. In each experiment we identified the total cell population on a forward scatter versus side scatter dot blot (A), followed by identification of the single-cell population P1 (B). For each antibody in every experiment the antibody specific signal was defined according to the IgG control (C-H). (GIF 111 kb)
S1 Table
Cerebral microvascular proteins up regulated both after GCI treated with vehicle and the MEK1/2 inhibitor U0126 (both compared to sham). Accession number and protein name is given along with the number of up regulated peptides the protein identification was based upon and the relative expressional increase of each protein based on the iTRAQ ratios (Log2). (PDF 116 kb)
S2 Table
Cerebral microvascular proteins down regulated both after GCI treated with vehicle and the MEK1/2 inhibitor U0126 (both compared to sham). Accession number and protein name is given along with the number of down regulated peptides the protein identification was based upon and the relative expressional decrease of each protein based on the iTRAQ ratios (Log2). (PDF 98 kb)
S3 Table
Proteins with a unique regulation in the cerebral microvasculature 72 h after GCI (vehicle) compared to sham. Accession number and protein name is given along with the number of up regulated peptides the protein identification was based upon and the relative expressional increase of each protein based on the iTRAQ ratios (Log2). (PDF 139 kb)
S4 Table
Proteins with a unique down regulation in the cerebral microvasculature 72 h after GCI (vehicle) compared to sham. Accession number and protein name is given along with the number of up regulated peptides the protein identification was based upon and the relative expressional decrease of each protein is given based on the iTRAQ ratios (Log2). (PDF 106 kb)
S5 Table
Proteins with a unique up regulation in the cerebral microvasculature 72 h after GCI treated with the MEK1/2 inhibitor U0126 compared to sham. Accession number and protein name is given along with the number of up regulated peptides the protein identification was based upon and the relative expressional increase of each protein based on the iTRAQ ratios (Log2). (PDF 158 kb)
S6 Table
Proteins with a unique down regulation in the cerebral microvasculature 72 h after GCI treated with the MEK1/2 inhibitor U0126 compared to sham. Accession number and protein name is given along with the number of down regulated peptides the protein identification was based upon and the relative expressional decrease of each protein based on the iTRAQ ratios (Log2). (PDF 120 kb)
Rights and permissions
About this article
Cite this article
Spray, S., Johansson, S.E., Edwards, A.V.G. et al. Alterations in the Cerebral Microvascular Proteome Expression Profile After Transient Global Cerebral Ischemia in Rat. J Mol Neurosci 61, 396–411 (2017). https://doi.org/10.1007/s12031-016-0875-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-016-0875-8