Abstract
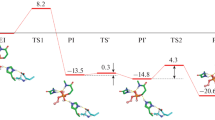
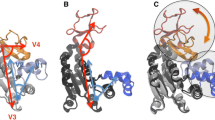
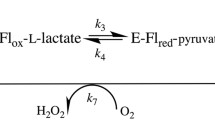
Cholinesterases display a hysteretic behavior with certain substrates and irreversible inhibitors. For years, this behavior has remained puzzling. However, several lines of evidence indicated that it is caused by perturbation of the catalytic triad and its water environment. In the present study, using molecular dynamics simulations of Ala328Cys BuChE mutant and wild-type BuChE in the absence and presence of a co-solvent (sucrose, glycerol), we provide evidence that hysteresis originates in a flip of the catalytic triad histidine (His438). This event is controlled by water molecules that interact with active site residues. The physiological significance of this phenomenon is still an issue.













Similar content being viewed by others
References
Badiou A, Froment MT, Fournier D, Masson P, Belzunces LP (2008) Hysteresis of insect acetylcholinesterase. Chem Biol Interact 175:410–412
Bakan A, Meireles LM, Bahar I (2011) ProDy: protein dynamics inferred from theory and experiments. Bioinformatics 27:1575–1577
Best RB, Zhu X, Shim J et al (2012) Optimization of the Additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ1 and χ2 dihedral angles. J Chem Theory Comput 8:3257–3273
Carletti E, Schopfer LM, Colletier J-P et al (2011) Reaction of cresyl saligenin phosphate, the organophosphorus implicated in the aerotoxic syndrome, with human cholinesterases: mechanistic studies employing kinetics, mass spectrometry and X-ray structure analysis. Chem Res Toxicol 24:797–808
Hatcher ER, Guvench O, MacKerell AD (2009) CHARMM additive all-atom force field for acyclic polyalcohols, acyclic carbohydrates, and inositol. J Chem Theory Comput 5:1315–1327
Hrabovska A, Debouzy JC, Froment MT, Devinsky F, Paulikova I, Masson P (2006) Rat butyrylcholinesterase-catalysed hydrolysis of N-alkyl homologues of benzoylcholine. FEBS J 273:1185–1197
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Khan MT (2009) Molecular interactions of cholinesterases inhibitors using in silico methods: current status and future prospects. New Biotechnol 25:331–346
Kwasnieski O, Verdier L, Malacria M, Derat E (2009) Fixation of the two tabun isomers in acetylcholinesterase: a QM/MM study. J Phys Chem B 113:10001–10007
Loudwig S, Nicolet Y, Masson P et al (2003) Photoreversible inhibition of cholinesterases: catalytic serine-labeled caged butyrylcholinesterase. Chembiochem 4:762–767
Lushchekina S, Grigorenko B, Morozov D, Polyakov I, Nemukhin A, Varfolomeev S (2010) Modeling of the mechanism of hydrolysis of succinylcholine in the active site of native and modified (Asp70Gly) human butyrylcholinesterase. Russ Chem Bull 59:55–60
Lushchekina S. V., Polomskih V. S., Varfolomeev S. D., Masson P. (2013) Molecular modeling of inhibition of butyrylcholinesterase by cresyl saligenin phosphate. Russian Chemical Bulletin. (In press)
Masson P (2012) Time-dependent kinetic complexities in cholinesterase-catalyzed reactions. Biochemistry (Mosc) 77:1147–1161
Masson P, Froment M-T, Bartels CF, Lockridge O (1996) Asp70 in the peripheral anionic site of human butyrylcholinesterase. Eur J Biochem 235:36–48
Masson P, Clery C, Guerra P, Redslob A, Albaret C, Fortier PL (1999) Hydration change during the aging of phosphorylated human butyrylcholinesterase: importance of residues aspartate-70 and glutamate-197 in the water network as probed by hydrostatic and osmotic pressures. Biochem J 343:361–369
Masson P, Froment MT, Fort S et al (2002) Butyrylcholinesterase-catalyzed hydrolysis of N-methylindoxyl acetate: analysis of volume changes upon reaction and hysteretic behavior. Biochim Biophys Acta 1597:229–243
Masson P, Goldstein BN, Debouzy JC, Froment MT, Lockridge O, Schopfer LM (2004) Damped oscillatory hysteretic behaviour of butyrylcholinesterase with benzoylcholine as substrate. Eur J Biochem 271:220–234
Masson P, Schopfer LM, Froment MT et al (2005) Hysteresis of butyrylcholinesterase in the approach to steady-state kinetics. Chem Biol Interact 157–158:143–152
Masson P, Froment MT, Gillon E, Nachon F, Darvesh S, Schopfer LM (2007) Kinetic analysis of butyrylcholinesterase-catalyzed hydrolysis of acetanilides. Biochim Biophys Acta 1774:1139–1147
Masson P, Lushchekina S, Schopfer LM, Lockridge O (2013) Effects of viscosity and osmotic stress on the reaction of human butyrylcholinesterase with cresyl saligenin phosphate, a toxicant related to the aerotoxic syndrome: kinetic and molecular dynamics studies. Biochem J 454:387–399
Morris GM, Huey R, Lindstrom W et al (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Nemukhin AV, Lushchekina SV, Bochenkova AV, Golubeva AA, Varfolomeev SD (2008) Characterization of a complete cycle of acetylcholinesterase catalysis by ab initio QM/MM modeling. J Mol Model 14:409–416
Ngamelue MN, Homma K, Lockridge O, Asojo OA (2007) Crystallization and X-ray structure of full-length recombinant human butyrylcholinesterase. Acta Crystallogr F 63:723–727
Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F (2003) Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J Biol Chem 278:41141–41147
Pedretti A, Villa L, Vistoli G (2004) VEGA—an open platform to develop chemo-bio-informatics applications, using plug-in architecture and script programming. J Comp Aided Mol Des 18:167–173
Peters J, Trovaslet M, Trapp M et al (2012) Activity and molecular dynamics relationship within the family of human cholinesterases. Phys Chem Chem Phys 14:6764–6770
Phillips JC, Braun R, Wang W et al (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802
Quinn DM (1987) Acetylcholinesterase: enzyme structure, reaction dynamics, and virtual transition states. Chem Rev 87:955–979
Raman EP, Guvench O, MacKerell AD (2010) CHARMM additive all-atom force field for glycosidic linkages in carbohydrates involving furanoses. J Phys Chem B 114:12981–12994
Sadovnichy V, Tikhonravov A, Voevodin V, Opanasenko V (2013) “Lomonosov”: supercomputing at Moscow State University. In: Vetter JS (ed) Contemporary high performance computing: from petascale toward exascale. CRC, Boca Raton, pp 283–307
Tai K, Shen T, Borjesson U, Philippopoulos M, McCammon JA (2001) Analysis of a 10-ns molecular dynamics simulation of mouse acetylcholinesterase. Biophys J 81:715–724
Valiev M, Bylaska EJ, Govind N et al (2010) NWChem: a comprehensive and scalable open-source solution for large scale molecular simulations. Comput Phys Commun 181:1477–1489
Word JM, Lovell SC, Richardson JS, Richardson DC (1999) Asparagine and glutamine: using hydrogen atom contacts in the choice of side-chain amide orientation. J Mol Biol 285:1735–1747
Acknowledgments
This work was partly supported by the Russian Foundation for Basic Research (project 12-03-31039-mol_a) and Fellowship of the President of Russia (СП-185.2012.4). We thank the Supercomputing Center of Lomonosov Moscow State University for providing computational facilities. We thank Prof. Vytas Švedas (Lomonosov Moscow State University) for valuable discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lushchekina, S.V., Nemukhin, A.V., Varfolomeev, S.D. et al. Molecular Modeling Evidence for His438 Flip in the Mechanism of Butyrylcholinesterase Hysteretic Behavior. J Mol Neurosci 52, 434–445 (2014). https://doi.org/10.1007/s12031-013-0178-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-013-0178-2