Abstract
Background
Hepatocellular carcinoma is one of the most common cancers and the second leading cause of cancer-related deaths worldwide. Only a small proportion of patients benefit from curative treatment and the prognosis is very poor for the majority of cases due to late presentation, resistance to chemotherapy and high recurrence rate. In recent years, progress in stem cell biology allowed us to explain that hierarchically organized cancer stem cells (CSCs) drive histological and functional heterogeneity of hematological malignancies and solid tumors.
Methods and Results
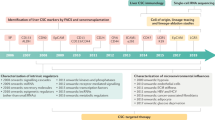
Also referred to as tumor-initiating cells, CSCs have been isolated from both hepatocellular carcinoma (HCC) cell lines and primary tumors by using hepatic progenitor markers. Although there is still no consensus on cancer stem cell phenotype in HCC, single or combined use of CSC markers defines a minor population of tumor cells with the capacity of self-renewing and the ability to recapitulate the original tumor heterogeneity.
Conclusions
This review focuses on the biological features of CSCs and their potential as diagnostic/prognostic tools and therapeutic targets in HCC.

Similar content being viewed by others
References
Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–8.
Reya T, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11.
Soltysova A, Altanerova V, Altaner C. Cancer stem cells. Neoplasma. 2005;52(6):435.
Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10(6):717–28.
Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197(4302):461–3.
Dick D. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature med. 1997;3(730–737):1.
Al-Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci. 2003;100(7):3983–8.
Dalerba P, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci. 2007;104(24):10158–63.
O’Brien CA, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–10.
Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–5.
Li C, et al. Identification of pancreatic cancer stem cells. Cancer res. 2007;67(3):1030–7.
Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401.
Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60.
Piccirillo S, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444(7120):761–5.
Chiba T, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell–like properties. Hepatology. 2006;44(1):240–51.
Haraguchi N, et al. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24(3):506–13.
Yang ZF, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13(2):153–66.
Zhang S, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer res. 2008;68(11):4311–20.
Curley MD, et al. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27(12):2875–83.
Sugihara E, Saya H. Complexity of cancer stem cells. Int J Cancer. 2013;132(6):1249–59.
Clevers H. The cancer stem cell: premises, promises and challenges. Nat med. 2011:313–9.
Zhou B-BS, et al. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat rev Drug Discov. 2009;8(10):806–23.
Patel P, Chen E. Cancer stem cells, tumor dormancy, and metastasis. Front Endocrinol. 2012;3:125.
Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15.
Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–51.
Torre LA, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
London W, McGlynn K. Liver cancer. Cancer Epidemiology and Prevention. 2006;3:763–86.
Jemal A, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Yeh MM. Pathology of combined hepatocellular-cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25(9):1485–92.
Ma S, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132(7):2542–56.
Suetsugu A, et al. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys res Commun. 2006;351(4):820–4.
Yamashita T, et al. Activation of hepatic stem cell marker EpCAM by Wnt–β-catenin signaling in hepatocellular carcinoma. Cancer res. 2007;67(22):10831–9.
Turner R, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology. 2011;53(3):1035–45.
Zhang L, et al. The stem cell niche of human livers: symmetry between development and regeneration. Hepatology. 2008;48(5):1598–607.
Tang Y, et al. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-β and IL-6 signaling. Proc Natl Acad Sci. 2008;105(7):2445–50.
Chen Y, et al. Mature hepatocytes exhibit unexpected plasticity by direct dedifferentiation into liver progenitor cells in culture. Hepatology. 2012;55(2):563–74.
Yin S, et al. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120(7):1444–50.
Yang ZF, et al. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008;47(3):919–28.
Yamashita T, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136(3):1012–1024. e4.
Haraguchi N, et al. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010;120(9):3326–39.
Yin AH, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90(12):5002–12.
Grosse-Gehling P, et al. CD133 as a biomarker for putative cancer stem cells in solid tumours: limitations, problems and challenges. J Pathol. 2013;229(3):355–78.
Zheng Y-W, et al. The CD133+ CD44+ precancerous subpopulation of oval cells is a therapeutic target for hepatocellular carcinoma. Stem Cells dev. 2014;23(18):2237–49.
Tang KH, et al. CD133+ liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology. 2012;55(3):807–20.
Marhaba R, Zöller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 2004;35(3):211–31.
Afify A, Purnell P, Nguyen L. Role of CD44s and CD44v6 on human breast cancer cell adhesion, migration, and invasion. Exp Mol Pathol. 2009;86(2):95–100.
van der Windt GJ, et al. CD44 is protective during hyperoxia-induced lung injury. Am J Respir Cell Mol Biol. 2011;44(3):377–83.
Zhu Z, et al. Cancer stem/progenitor cells are highly enriched in CD133+ CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010;126(9):2067–78.
Reif AE, Allen JM. The AKR thymic antigen and its distribution in leukemias and nervous tissues. J Exp med. 1964;120(3):413–33.
Mima K, et al. CD44s regulates the TGF-β–mediated mesenchymal phenotype and is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer res. 2012;72(13):3414–23.
Ishimoto T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc− and thereby promotes tumor growth. Cancer Cell. 2011;19(3):387–400.
Lu J-W, et al. Overexpression of Thy1/CD90 in human hepatocellular carcinoma is associated with HBV infection and poor prognosis. Acta Histochem. 2011;113(8):833–8.
Went PT, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35(1):122–8.
Kim JW, et al. Cancer-associated molecular signature in the tissue samples of patients with cirrhosis. Hepatology. 2004;39(2):518–27.
Yamashita T, et al. Discrete nature of EpCAM+ and CD90+ cancer stem cells in human hepatocellular carcinoma. Hepatology. 2013;57(4):1484–97.
Kurtz J-E, Dufour P. Adecatumumab: an anti-EpCAM monoclonal antibody, from the bench to the bedside. Expert Opin Biol Ther. 2010;10(6):951–8.
Gires O, Bauerle PA. EpCAM as a target in cancer therapy. J Clin Oncol. 2010;28(15):e239–40.
Mina-Osorio P. The moonlighting enzyme CD13: old and new functions to target. Trends Mol med. 2008;14(8):361–71.
Kim HM, et al. Increased CD13 expression reduces reactive oxygen species, promoting survival of liver cancer stem cells via an epithelial–mesenchymal transition-like phenomenon. Ann Surg Oncol. 2012;19(3):539–48.
Acknowledgements
The study was supported by a grant of The Scientific and Technological Research Council of Turkey (TUBITAK) to Tamer Yagci (111S484). The authors apologize to those investigators whose meritorious works were not cited due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yagci, T., Cetin, M. & Ercin, P.B. Cancer Stem Cells in Hepatocellular Carcinoma. J Gastrointest Canc 48, 241–245 (2017). https://doi.org/10.1007/s12029-017-9960-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-017-9960-7