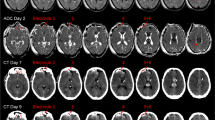
Abstract
Background
How widely spreading depolarizations (SDs) propagate through the gyrencephalic brain, including sulci and deeper cortical areas, remains an important clinical question. Here, we investigated SDs that occur spontaneously after subarachnoid placement of autologous blood clots in sulci of the juvenile swine brain.
Methods
To investigate the three-dimensional spread of waves, animals underwent continuous diffusion-weighted magnetic resonance imaging (DW-MRI) for up to 6 h following clot placement. SD is the mechanism of the cytotoxic edema of developing infarction that is diagnosed by DW-MRI, and DW-MRI also captures transient diffusion restriction caused by SD in less injured or healthy brains. Here, images (b = 0, 375, and 750) were acquired across five coronal slices with 1.25 × 1.25-mm in-plane resolution and 5-mm slice thickness, and the protocol was repeated every 6.83–9.15 s. Spatial drift correction, temporal smoothing, and signal intensity normalization were applied to generate videos of diffusion signal intensity changes for each coronal slice.
Results
Review of video data from five animals revealed ten discrete events consisting of focal diffusion restriction that propagated through cerebral cortex. All events originated in the cortex surrounding the sulcal clot, either in the gyrus (n = 4) or in the sulcal depth (n = 6). In six cases, two to three independent waves spread simultaneously in medial, lateral, and antero–posterior directions. Waves traveled within sulcal walls, traversed the depths of sulci to re-emerge on the adjacent gyrus, and, in three cases, spread fully around the dorsolateral convexity. One event spread deep to olfactory regions along midline cortex, and no events were observed contralateral to the subarachnoid clot.
Conclusions
Together, these results suggest that SDs in the injured gyrencephalic brain originate near the injury focus and can spread extensively through the cortex to wide and deep uninjured regions. These findings have implications for transient neurologic deficits in the neurocritically ill patient and relevance to patient monitoring and therapeutics.


Similar content being viewed by others
References
Kaufmann D, Theriot JJ, Zyuzin J, et al. Heterogeneous incidence and propagation of spreading depolarizations. J Cereb Blood Flow Metab. 2016. https://doi.org/10.1177/0271678X16659496.
Santos E, Scholl M, Sanchez-Porras R, et al. Radial, spiral and reverberating waves of spreading depolarization occur in the gyrencephalic brain. Neuroimage. 2014;99C:244–55.
Santos E, Sanchez-Porras R, Sakowitz OW, et al. Heterogeneous propagation of spreading depolarizations in the lissencephalic and gyrencephalic brain. J Cereb Blood Flow Metab. 2017;37:2639–43.
Dreier JP, Lemale CL, Kola V, et al. Spreading depolarization is not an epiphenomenon but the principal mechanism of the cytotoxic edema in various gray matter structures of the brain during stroke. Neuropharmacology. 2018;134:189–207.
Hartings JA, Shuttleworth CW, Kirov SA, et al. The continuum of spreading depolarizations in acute cortical lesion development: examining Leao’s legacy. J Cereb Blood Flow Metab. 2017;37:1571–94.
Van Harreveld A, Khattab FI. Changes in cortical extracellular space during spreading depression investigated with the electron microscope. J Neurophysiol. 1967;30:911–29.
Kirov SA, Fomitcheva IV, Sword J. Rapid neuronal ultrastructure disruption and recovery during spreading depolarization-induced cytotoxic edema. Cereb Cortex. 2020;30:5517–31.
Murphy TH, Li P, Betts K, et al. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J Neurosci. 2008;28:1756–72.
Risher WC, Ard D, Yuan J, et al. Recurrent spontaneous spreading depolarizations facilitate acute dendritic injury in the ischemic penumbra. J Neurosci. 2010;30:9859–68.
Latour LL, Hasegawa Y, Formato JE, et al. Spreading waves of decreased diffusion coefficient after cortical stimulation in the rat brain. Magn Reson Med. 1994;32:189–98.
de Crespigny A, Rother J, van Bruggen N, et al. Magnetic resonance imaging assessment of cerebral hemodynamics during spreading depression in rats. J Cereb Blood Flow Metab. 1998;18:1008–17.
Umesh Rudrapatna S, Hamming AM, Wermer MJ, et al. Measurement of distinctive features of cortical spreading depolarizations with different MRI contrasts. NMR Biomed. 2015;28:591–600.
James MF, Smith MI, Bockhorst KH, et al. Cortical spreading depression in the gyrencephalic feline brain studied by magnetic resonance imaging. J Physiol. 1999;519(Pt 2):415–25.
Busch E, Gyngell ML, Eis M, et al. Potassium-induced cortical spreading depressions during focal cerebral ischemia in rats: contribution to lesion growth assessed by diffusion-weighted NMR and biochemical imaging. J Cereb Blood Flow Metab. 1996;16:1090–9.
Kastrup A, Neumann-Haefelin T, Moseley ME, et al. High speed diffusion magnetic resonance imaging of ischemia and spontaneous periinfarct spreading depression after thromboembolic stroke in the rat. J Cereb Blood Flow Metab. 2000;20:1636–47.
Busch E, Beaulieu C, de Crespigny A, et al. Diffusion MR imaging during acute subarachnoid hemorrhage in rats. Stroke. 1998;29:2155–61.
Yenari MA, Onley D, Hedehus M, et al. Diffusion- and perfusion-weighted magnetic resonance imaging of focal cerebral ischemia and cortical spreading depression under conditions of mild hypothermia. Brain Res. 2000;885:208–19.
Hartings JA, York J, Carroll CP, et al. Subarachnoid blood acutely induces spreading depolarizations and early cortical infarction. Brain. 2017;140:2673–90.
Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56.
Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41.
Bowyer SM, Tepley N, Papuashvili N, et al. Analysis of MEG signals of spreading cortical depression with propagation constrained to a rectangular cortical strip. II. Gyrencephalic swine model. Brain Res. 1999;843:79–86.
Sloan N, Jasper H. The identity of spreading depression and “suppression.” EEG Clin Neurophyiol. 1950;2:59–78.
Woitzik J, Hecht N, Pinczolits A, et al. Propagation of cortical spreading depolarization in the human cortex after malignant stroke. Neurology. 2013;80:1095–102.
Hadjikhani N, Sanchez Del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687–92.
von Bornstadt D, Houben T, Seidel JL, et al. Supply-demand mismatch transients in susceptible peri-infarct hot zones explain the origins of spreading injury depolarizations. Neuron. 2015;85:1117–31.
Kumagai T, Walberer M, Nakamura H, et al. Distinct spatiotemporal patterns of spreading depolarizations during early infarct evolution: evidence from real-time imaging. J Cereb Blood Flow Metab. 2011;31:580–92.
Hartings JA. Spreading depolarization monitoring in neurocritical care of acute brain injury. Curr Opin Crit Care. 2017;23:94–102.
Dreier JP, Fabricius M, Ayata C, et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: review and recommendations of the COSBID research group. J Cereb Blood Flow Metab. 2017;37:1595–625.
Robinson D, Hartings J, Foreman B. First report of spreading depolarization correlates on scalp EEG confirmed with a depth electrode. Neurocrit Care. 2021;35(Suppl 2):100–4.
Funding
This work was supported by the American Association of Neurological Surgeons/Congress of Neurological Surgeons (AANS/CNS) Joint Section on Cerebrovascular Surgery Robert J. Dempsey, MD, Cerebrovascular Research Award (to CPC) and the Mayfield Education and Research Foundation (to JAH, CPC).
Author information
Authors and Affiliations
Contributions
JAH conceived the study, contributed to experimental design and execution, drafted the manuscript, and approval the final manuscript version. CPC contributed to experimental design, performed surgeries, collected data, edited the manuscript, and approved the final manuscript version. GL designed and executed the magnetic resonance imaging protocols, processed the data, analyzed results, contributed to manuscript preparation, and approved the final manuscript version.
Corresponding author
Ethics declarations
Copyright Statement
Christopher P. Carroll, LCDR, MC, USN, is a military service member. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that "Copyright protection under this title is not available for any work of the United States Government." Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties.
Disclaimer
The views expressed in this article reflect the results of research conducted by the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or United States Government.
Conflicts of Interest
The authors have no other conflicts to disclose.
Ethical Approval/Animal Rights
This study complies with all ethical guidelines and was conducted with approval and supervision of the institutional animal care and use committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the collection “Spreading Cortical Depolarization.”
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 330 kb)
Rights and permissions
About this article
Cite this article
Hartings, J.A., Carroll, C.P. & Lee, G. Spreading Diffusion-Restriction Events in the Gyrencephalic Brain After Subarachnoid Hemorrhage Revealed by Continuous Magnetic Resonance Imaging. Neurocrit Care 37 (Suppl 1), 60–66 (2022). https://doi.org/10.1007/s12028-021-01376-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01376-0