Abstract
Background
Vagus nerve stimulation (VNS) during post-resuscitation may increase recovery of cerebral blood flow (CBF) and reduce neurological injury.
Objective
This study was designed to investigate the effect of electrical VNS on neurological outcomes following cardiac arrest (CA).
Methods
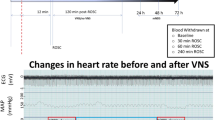
Male Sprague–Dawley rats (n = 48) were subjected to the asphyxial CA model and blindly allocated to the VN isolation (CA + VN isolation) or VNS group (CA + VNS group). Cardiopulmonary resuscitation was initiated 450 s after pulseless electrical arrest, and the left cervical vagus nerve was electrically stimulated (0.05 mA, 1 Hz) for 3 h in the CA + VNS group. The neurological deficit score (NDS) and overall performance category (OPC) were assessed at 24 h after resuscitation, and histological injury of the hippocampus was evaluated. Independent experiments were performed to evaluate the effect of VNS on global cortical CBF after resuscitation using laser speckle Doppler imaging through a thinned skull window from pre-arrest to 6 h after resuscitation.
Results
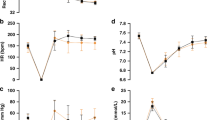
The baseline characteristics were not significantly different between the two groups. The NDS was significantly higher, and the OPC was substantially lower in the CA + VNS group (p = 0.022 and p = 0.049, respectively) supported by decrease in histological injury of the hippocampal CA1 region. CBF in the early period of post-return of spontaneous circulation (ROSC) was significantly higher in the CA + VNS group (p < 0.05 at post-ROSC 2 h and 4 h), and 4-hydroxynonenal was significantly lower in the CA + VNS group (p = 0.026).
Conclusions
VNS improved cerebral perfusion and neurological outcomes at 24 h after ROSC in an asphyxial CA model of rats.





Similar content being viewed by others
References
Nolan JP, Neumar RW, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79(3):350–79.
Mangus DB, Huang L, Applegate PM, et al. A systematic review of neuroprotective strategies after cardiac arrest: from bench to bedside (part I—protection via specific pathways). Med Gas Res. 2014;4:9.
Huang L, Applegate PM, Gatling JW, et al. A systematic review of neuroprotective strategies after cardiac arrest: from bench to bedside (part II—comprehensive protection). Med Gas Res. 2014;4:10.
Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369(23):2197–206.
Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63.
Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56.
Iordanova B, Li L, Clark RSB, Manole MD. Alterations in cerebral blood flow after resuscitation from cardiac arrest. Front Pediatr. 2017;5:174.
van den Brule JM, Vinke EJ, van Loon LM, van der Hoeven JG, Hoedemaekers CW. Low spontaneous variability in cerebral blood flow velocity in non-survivors after cardiac arrest. Resuscitation. 2017;111:110–5.
Chen WL, Tsai TH, Huang CC, Chen JH, Kuo CD. Heart rate variability predicts short-term outcome for successfully resuscitated patients with out-of-hospital cardiac arrest. Resuscitation. 2009;80(10):1114–8.
Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–62.
Jiang Y, Li L, Liu B, et al. Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS ONE. 2014;9(7):e102342.
Ay I, Nasser R, Simon B, Ay H. Transcutaneous cervical vagus nerve stimulation ameliorates acute ischemic injury in rats. Brain Stimul. 2016;9(2):166–73.
Jiang Y, Li L, Ma J, et al. Auricular vagus nerve stimulation promotes functional recovery and enhances the post-ischemic angiogenic response in an ischemia/reperfusion rat model. Neurochem Int. 2016;97:73–82.
Zhang L, Ma J, Jin X, et al. L-PGDS mediates vagus nerve stimulation-induced neuroprotection in a rat model of ischemic stroke by suppressing the apoptotic response. Neurochem Res. 2017;42(2):644–55.
Sun P, Wang J, Zhao S, et al. Improved outcomes of cardiopulmonary resuscitation in rats treated with vagus nerve stimulation and its potential mechanism. Shock. 2018;49(6):698–703.
Zobel A, Joe A, Freymann N, et al. Changes in regional cerebral blood flow by therapeutic vagus nerve stimulation in depression: an exploratory approach. Psychiatry Res. 2005;139(3):165–79.
Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol. 2014;592(5):841–59.
National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academies Press; 2011.
Lee JH, Kim K, Jo YH, et al. Effect of valproic acid on survival and neurologic outcomes in an asphyxial cardiac arrest model of rats. Resuscitation. 2013;84(10):1443–9.
Jia X, Koenig MA, Shin HC, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation. 2008;76(3):431–42.
Winship IR. Laser speckle contrast imaging to measure changes in cerebral blood flow. Methods Mol Biol. 2014;1135:223–35.
Buunk G, van der Hoeven JG, Meinders AE. Cerebral blood flow after cardiac arrest. Neth J Med. 2000;57(3):106–12.
He J, Lu H, Young L, et al. Real-time quantitative monitoring of cerebral blood flow by laser speckle contrast imaging after cardiac arrest with targeted temperature management. J Cereb Blood Flow Metab. 2017;2017:271678X17748787.
Manole MD, Kochanek PM, Bayir H, et al. Brain tissue oxygen monitoring identifies cortical hypoxia and thalamic hyperoxia after experimental cardiac arrest in rats. Pediatr Res. 2014;75(2):295–301.
Henry TR, Votaw JR, Pennell PB, et al. Acute blood flow changes and efficacy of vagus nerve stimulation in partial epilepsy. Neurology. 1999;52(6):1166–73.
Pardo JV, Sheikh SA, Schwindt GC, et al. Chronic vagus nerve stimulation for treatment-resistant depression decreases resting ventromedial prefrontal glucose metabolism. Neuroimage. 2008;42(2):879–89.
Rutecki P. Anatomical, physiological, and theoretical basis for the antiepileptic effect of vagus nerve stimulation. Epilepsia. 1990;31(Suppl 2):S1–6.
Parada E, Egea J, Buendia I, et al. The microglial alpha7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid Redox Signal. 2013;19(11):1135–48.
Miyamoto O, Pang J, Sumitani K, et al. Mechanisms of the anti-ischemic effect of vagus nerve stimulation in the gerbil hippocampus. NeuroReport. 2003;14(15):1971–4.
Follesa P, Biggio F, Gorini G, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34.
Kim C, Fahrenbruch CE, Cobb LA, Eisenberg MS. Out-of-hospital cardiac arrest in men and women. Circulation. 2001;104(22):2699–703.
Acknowledgements
We acknowledge the technical assistance of animal care provided by pre-clinical experimental center in SNUBH.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03034360).
Author information
Authors and Affiliations
Contributions
JHL and YHJ designed the study and prepared the protocol. BK, IP, SK, MJL, and JHL performed experiments. All authors participated in interpretation of data. BK, IP, and JHL drafted and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
The Institutional Animal Care and Use Committee of Seoul National University Bundang Hospital (protocol no. BA1705-223/042-01) approved this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, B., Park, I., Lee, J.H. et al. Effect of Electrical Vagus Nerve Stimulation on Cerebral Blood Flow and Neurological Outcome in Asphyxial Cardiac Arrest Model of Rats. Neurocrit Care 30, 572–580 (2019). https://doi.org/10.1007/s12028-018-0640-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-018-0640-7