Abstract
Background
Early diagnostic orientation for differentiating pneumonia from pneumonitis at the early stage after aspiration would be valuable to avoid unnecessary antibiotic therapy. We assessed the accuracy of procalcitonin (PCT) in diagnosing aspiration pneumonia (AP) in intensive care unit (ICU) patients requiring mechanical ventilation after out-of-hospital coma.
Methods
Prospective observational 2-year cohort study in a medical-surgical ICU. PCT, C-reactive protein (CRP) and white blood cell count (WBC) were measured at admission (H0) and 6 h (H), H12, H24, H48, H96, and H120 after inclusion. Lower respiratory tract microbiological investigations performed routinely in patients with aspiration syndrome were the reference standard for diagnosing AP. Performance of PCT, CRP, and WBC up to H48 in diagnosing AP was compared based on the areas under the ROC curves (AUC) and likelihood ratios (LR+ and LR−) computed for the best cutoff values.
Results
Of 103 patients with coma, 45 (44%) had AP. Repeated PCT assays demonstrated a significant increase in patients with AP versus without AP from H0 to H120. Among the three biomarkers, PCT showed the earliest change. ROC-AUC values were poor for all three biomarkers. Best ROC-AUC values for diagnosing AP were for CRP at H24 [0.73 (95%CI 0.61–0.84)] and PCT at H48 [0.73 (95%CI 0.61–0.84)]. LR+ was best for PCT at H24 (3.5) and LR− for CRP and WBC at H24 (0.4 and 0.4, respectively).
Conclusions
Early and repeated assays of PCT, CRP, and WBC demonstrated significant increases in all three biomarkers in patients with versus without AP. All three biomarkers had poor diagnostic performance for ruling out AP. Whereas PCT had the fastest kinetics, PCT assays within 48 h after ICU admission do not help to diagnose AP in ICU patients with coma.
Similar content being viewed by others
Introduction
Aspiration syndrome occurs in as many as 20–60% of critically ill patients with coma requiring mechanical ventilation [1,2,3]. This syndrome may result in either of two entities that must be distinguished, as their therapeutic implications differ [4]. One is aspiration pneumonitis, also known as Mendelson’s syndrome, in which the aspiration contents cause chemical damage to the airways and lung parenchyma [5]. The other is AP, which is a lung infection due chiefly to the bacteria present in the oropharyngeal secretions [6].
The diagnosis of AP is obvious when a microbiological lung sample taken 48 h after aspiration syndrome yields positive cultures. However, pneumonia requires treatment before the culture results are available. The respiratory symptoms and radiological signs lack sensitivity for differentiating pneumonia from pneumonitis. As a result, avoiding unnecessary antibiotic therapy is challenging at the early stage [7], and routine prophylactic antibiotic therapy in comatose patients has been advocated [8]. Early diagnostic orientation would therefore be valuable. Biomarkers might help to diagnose AP if they change rapidly in response to the infection and show strong predictive performance [9]. Few studies of this possibility are available, however, and their results are conflicting, particularly regarding serum PCT levels as a tool for supporting a diagnosis of AP. The poor performance of PCT might be related either to rapid short-lived changes being missed by delayed assaying or to the characteristics of the study populations [10,11,12,13].
Here, we conducted a prospective single-center study comparing early and repeated assays of PCT, CRP, and WBC as tools capable of ruling out AP in patients with coma requiring mechanical ventilation.
Materials and Methods
The appropriate ethics committee (Comité de Protection des Personnes de Saint-Germain-en-Laye) approved this prospective observational study (No. 08049). Written informed consent was obtained from surrogates before study inclusion. Written consent was then requested from all included patients as soon as they regained competence. If the surrogates chose not to give consent, the patients were not included in the analysis.
Patients
Adults admitted to our intensive care unit (ICU) during the 3-year period between 2008 and 2010 were included prospectively if they experienced a coma requiring out-of-hospital initiation of mechanical ventilation. Coma was defined as a Glasgow Coma Scale (GCS) score lower than 9. We did not include patients with arousal disorders and a GCS score ≥ 9, in-hospital onset of coma, post-anoxic coma, or coma with evidence of infection other than AP.
Study Design and Management
After a careful history and thorough physical examinations on-scene and at ICU admission, including a neurological evaluation, investigations were performed as appropriate to identify the factors causing the coma. Laboratory tests were obtained routinely. Plasma anticonvulsant drug assays and qualitative tests for toxic substances or medications known to cause coma were performed at the discretion of the attending physicians. Cerebral imaging and electroencephalography (EEG) monitoring were obtained routinely. Lumbar puncture was performed when there was a fever or clinical suspicion of central nervous system infection and when deemed appropriate by the attending physicians. According to our inclusion criteria, patients with meningitis or encephalitis were not eligible for inclusion. The primary cause of coma was classified as drug poisoning, stroke, traumatic brain injury, or convulsive status epilepticus. All patients received early life-supporting interventions as needed, variously combining vasoactive drugs, fluid resuscitation, and renal replacement therapy, in addition to mechanical ventilation. Standard medical treatment for the suspected cause was introduced immediately after the initial clinical evaluation.
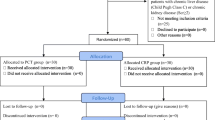
As illustrated in Fig. 1, in addition to supportive and etiological management, all included patients received a standardized management strategy aimed at identifying and promptly treating AP. Patients with suspected AP underwent blind bronchial sampling and/or tracheal aspirate sampling for microbiological studies. In the absence of contraindications and of risk factors for resistance, amoxicillin/clavulanic acid was started immediately after lung-sample collection; this treatment was continued for a total of 7 days if the cultures were positive and stopped otherwise.
For the study, blood samples were drawn at inclusion then 6, 12, 24, 48, 72, 96, and 120 h (H) later for assays of PCT, CRP, and WBC. PCT was assayed in 100-µL serum samples by two investigators (AM and MB), working independently of each other and blinded to the clinical data, using a time-resolved amplified cryptate emission technology on a Kryptor analyzer (Brahms Diagnostica, Berlin, Germany). The bedside physicians were blinded to the PCT assay results.
Definitions
Aspiration syndrome was defined as a radiographic pulmonary infiltrate within 48 h after ICU admission combined with at least one major criterion (sputum production, fever) or two minor criteria (dyspnea, pleuritic chest pain, altered mental status, pulmonary consolidation on physical examination).
AP was defined as a positive lung-sample culture in a patient with aspiration syndrome. A positive lung-sample culture was defined as a blind bronchial sample ≥ 103 CFU and/or a tracheal aspirate sample ≥ 106 CFU.
Data Collection
A standardized form was used to collect the variables listed in Tables 1 and 2 and in Supplemental Tables 1 and 2. Clinical features and criteria for suspected aspiration syndrome were collected prospectively at the bedside. Acute illness severity and organ dysfunction at ICU admission were assessed using the Simplified Acute Physiology Score II (SAPS II) and the Sequential Organ Failure Assessment (SOFA) score.
Statistical Analysis
Quantitative parameters were described as median (interquartile range, IQR) or mean (standard deviation, SD) and qualitative parameters as number (percentage). We compared categorical variables using Fisher’s exact test and continuous variables using Wilcoxon’s rank-sum test. Receiver-operating characteristic (ROC) curves were plotted to evaluate the performance of PCT, CRP, and WBC for diagnosing AP at H6, H12, H24, and H48 after inclusion (H0). The area under each ROC curve (ROC-AUC) was calculated, and its 95% confidence interval (95%CI) was estimated as described by Delong and Delong. When ROC-AUC was greater than 0.6, several cutoffs were evaluated and the cutoff providing the best compromise between sensitivity (Se) and specificity (Sp) was identified. When several points were at the same distance from the ideal curve (Se = 1 and Sp = 1), priority was given to specificity. The positive and negative likelihood ratios (LR+ and LR−) computed for the optimal cutoffs were calculated. LR values above 10 strongly support presence of the disease, whereas LR values less than 0.1 strongly support absence of the disease. Point estimates and 95%CIs were determined. Analyses were performed using R software 2.15.0 (http://r-project.org).
Results
Of the 371 patients admitted to the ICU for coma during the 3-year study period, 268 did not meet the inclusion criteria, leaving 103 patients for the study (Supplemental Figure).
Study Population
Among the 103 included patients with coma (Table 1), 81 (78.6%) had suspected aspiration syndrome within 48 h after inclusion. Criteria for suspecting aspiration syndrome were as follows: radiographic pulmonary infiltrate, n = 84 (82%); sputum production, n = 79 (77%); and fever, n = 66 (64%). These 81 patients underwent lung sampling, which yielded positive cultures in 45 (55%) patients. Thus, AP was diagnosed in 45/103 (44%) of included patients. The main microorganisms identified were as follows: Streptococcus pneumonia n = 16; methicillin-sensitive Staphylococcus aureus, n = 10; Haemophilus influenzae, n = 6; various streptococci, n = 6; various Gram-negative bacilli, n = 4; and oral saprophytic bacteria, n = 14; some patients had more than one microorganism identified. Supplemental Table 1 reports temperature measurements in patients with and without bacterial AP over the first 120 h after study inclusion.
Kinetics of PCT, CRP and WBC
Supplemental Table 2 reports and Fig. 2 illustrates the PCT, CRP, and WBC values in patients with and without AP. Repeated PCT assays demonstrated a significant increase in patients with versus without AP, from inclusion (H0) to H120. The PCT level peaked at H12 and then declined gradually. CRP increased in both groups from H6 to H48, but the increase was larger in the group with AP; the value decreased thereafter but remained above normal at H120. WBC was elevated at ICU admission in both groups, and then increased significantly to a peak at H24 in the group with AP. PCT increased and decreased more rapidly than did CRP, while WBC remained high throughout the evaluation period.
PCT, CRP, and WBC kinetics over the first 120 h after study inclusion. H0, H6, H12, H24, H48, H72, H96, and H120, where H means hour(s), are the times samples drawn to assay the three biomarkers. H0 was study inclusion, which occurred at ICU admission. The plots are the mean values, and the bars indicate the 95% confidence intervals of the means. Blue lines indicate the group with AP and black lines the group without AP
Diagnostic Performance of PCT, CRP and WBC
Table 2 reports and Fig. 3 illustrates the performance of PCT, CRP, and WBC for diagnosing AP. ROC-AUC values were poor for all three biomarkers over the first 48 h after study inclusion. The best ROC-AUC values were observed at H24 for CRP [0.73 (95%CI 0.61–0.84)] and at H48 for PCT [0.73 (95%CI 0.61–0.84)], respectively. The LR+ and LR− values computed for the best cutoffs indicated poor diagnostic performance. LR+ values were low for all three biomarkers, with the best value being for PCT at H24 (3.5). LR− was best for CRP and WBC and was lowest at H24 but nevertheless far greater than 0.1 (0.4 for CRP and 0.4 for WBC).
ROC curves of the performance of PCT, CRP, and WBC for diagnosing AP over the first 48 h after study inclusion. H0 (Panel A), H6 (Panel B), H12 (Panel C), H24 (Panel D), and H48 (Panel E) where H means hour(s), are the times samples drawn to assay the three biomarkers. H0 was study inclusion, which occurred at ICU admission. In each panel, the black line is the ROC curve for PCT, the red line the ROC curve for CRP, and the blue line the ROC curve for the WBC. PCT procalcitonin, CRP C-reactive protein, WBC white blood cell count
Discussion
Of the 103 critically ill patients with a coma requiring mechanical ventilation who were included in this prospective study, 44% received a diagnosis of AP. Early and repeated assays of PCT, CRP, and WBC demonstrated significant increases in all three biomarkers in patients with versus without AP. PCT had the fastest kinetics. However, all three biomarkers had poor diagnostic performance for ruling out AP, with LR− values far greater than 0.1.
The literature abounds in studies of PCT as a diagnostic or prognostic aid and even as a tool for guiding treatment decisions, particularly in patients with lower respiratory tract infections [14,15,16,17,18,19]. However, few studies have assessed PCT for diagnosing AP in patients from the community. The earliest of these studies showed poor diagnostic performance in 65 patients with consciousness impairment in the ICU [10]. However, the population differed substantially from ours. Disease severity was markedly worse, with a mean APACHE II score of 28.0 ± 5.7; the risk of aspiration was lower, as the mean GCS score was 10.3 ± 2.5; neurological causes of coma predominated; and most patients had been transferred to the ICU from healthcare facilities [10]. As indicated by high SAPS II and SOFA score values and low GCS scores at inclusion, our study provides a realistic picture of the presentation, management, and diagnosis of AP in ICU patients with out-of-hospital onset of coma [6]. The underlying diseases identified in our study were as expected in critically ill patients with coma and aspiration syndrome. As previously reported, the predominance of females may be related to the high proportion of patients with drug poisoning as the cause of coma [1, 7]. Our use of postanoxic coma as an exclusion criterion may explain some discrepancies with other studies. However, in patients with postanoxic coma, the previously reported association between PCT and postcardiac arrest syndrome might interfere with the assessment of PCT as a tool for diagnosing infection [12, 13].
Our results show that, of the three biomarkers, PCT levels performed best for diagnosing AP at the early stage. Thus, the PCT assays at H0 and H6 were associated with ROC-AUCs of 0.63 (0.52–0.74) and 0.69 (0.58–0.80), respectively, versus 0.58 (0.47–0.69) and 0.64 (0.52–0.75) for CRP and 0.53 (0.42–0.65) and 0.54 (0.41–0.66) for WBC. More interestingly, PCT was the biomarker with the earliest increase. Comparisons with the literature are difficult to draw, due to the paucity of studies in similar populations. The earliest study, as mentioned above, found no difference in PCT levels between patients with and without AP [10]. The ROC-AUC was 0.59 (0.47–0.92) in their study after the first 24 h of management. Thus, the ROC-AUCs in this earlier study and in ours were low. In contrast, high ROC-AUCs for PCT have been reported in other infections: 0.80 to 0.93 in community-acquired pneumonia [20, 21], 0.70 (0.60–0.80) in patients requiring mechanical ventilation [22] and 0.87 in those with ventilator-associated pneumonia [23,24,25].
One of the strengths of our study is the evaluation of biomarker kinetics. This evaluation showed that early PCT assays were associated with better discrimination between patients with and without AP and, also, that PCT levels decreased rapidly after treatment initiation. However, compared to other conventional markers for inflammation, PCT showed only moderate usefulness beyond the first 6–12 h after inclusion. CRP had low initial values and peaked only after 48 h. Finally, WBC increased significantly over time patients with AP, but the values were not clinically relevant. The performance parameters computed for the best cutoff values at various timepoints indicated only modest usefulness at best. For PCT, the cutoff of 0.07 ng/mL at study inclusion was associated with 54.5% Se, 67.9% Sp, 57.1% positive predictive value, 65.5% negative predictive value, an LR+ of 1.7 and an LR− of 0.7. Corresponding values at H6 for a PCT cutoff of 0.09 ng/mL were 64.3%, 67.3%, 60%, 71.2%, 2, and 0.5. PCT performed best helping to diagnosing AP at H12 and H24, demonstrating LR+ values of 2.3 and 3.5, respectively. However, at the same timepoints, as indicating by their LR− values, performance criteria were better for CRP than for PCT for ruling out AP. At H48, PCT had an LR− of 0.4, but at this timepoint the results of the microbiological studies are often already available. In other words, even if PCT values demonstrated significant differences between patients with and without AP, diagnostic performances were low in ruling out AP when comparing PCT to the other biomarkers based on their likelihood ratios (LR+ and LR−). Given these findings, we could not recommend deciding institution of antibiotics according to PCT values in patients with coma and possible AP.
Our study has several limitations. First, the applicability of our results to the full spectrum of ICU patients with coma is unclear. We included a uniform population of patients with coma onset in the community setting and etiologies were limited to stroke, traumatic brain injury, convulsive status epilepticus and drug poisoning. Both factors may limit generalizability. Moreover, similar investigations would be required in patients with coma onset in healthcare facilities. Second, one might argue that lung samples should have been obtained routinely in all included patients and not only in those with suspected aspiration syndrome. However, our approach was pragmatic, and as we wanted to maximize the pre-test probability we obtained lung samples only in patients with suspected AP. Third, unfortunately our study was not designed to help guiding of discontinuation of antibiotics and no recommendation can be formulated in that direction arising from our findings. Finally, although we computed LR+ and LR−, allowing not considering the prevalence of AP in this single-center prospective study, we cannot rule out that results would be different in a larger multicenter population.
In conclusion, PCT, compared to CRP and WBC, within 48 h after ICU admission of patients with out-of-hospital coma onset does not help to identify patients with AP.
References
Leroy O, Vandenbussche C, Coffinier C, Bosquet C, Georges H, Guery B, et al. Community-acquired aspiration pneumonia in intensive care units. Epidemiological and prognosis data. Am J Respir Crit Care Med. 1997;156(6):1922–9.
Bronchard R, Albaladejo P, Brezac G, Geffroy A, Seince PF, Morris W, et al. Early onset pneumonia: risk factors and consequences in head trauma patients. Anesthesiology. 2004;100(2):234–9.
Lepelletier D, Roquilly A, Demeure dit latte D, Mahe PJ, Loutrel O, Champin P, et al. Retrospective analysis of the risk factors and pathogens associated with early-onset ventilator-associated pneumonia in surgical-ICU head-trauma patients. J Neurosurg Anesthesiol. 2010;22(1):32–7.
Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–71.
Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191–205.
Adnet F, Baud F. Relation between Glasgow Coma Scale and aspiration pneumonia. Lancet. 1996;348(9020):123–4.
Lascarrou JB, Lissonde F, Le Thuaut A, Bachoumas K, Colin G, Henry Lagarrigue M, et al. Antibiotic therapy in comatose mechanically ventilated patients following aspiration: differentiating pneumonia from pneumonitis. Crit Care Med. 2017;45(8):1268–75.
Righy C, do Brasil PEA, Valles J, Bozza FA, Martin-Loeches I. Systemic antibiotics for preventing ventilator-associated pneumonia in comatose patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7(1):67.
Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(5):426–35.
El-Solh AA, Vora H, Knight PR 3rd, Porhomayon J. Diagnostic use of serum procalcitonin levels in pulmonary aspiration syndromes. Crit Care Med. 2011;39(6):1251–6.
Pusch F, Wildling E, Freitag H, Weinstabl C. Procalcitonin as a diagnostic marker in patients with aspiration after closed head injury. Wien Klin Wochenschr. 2001;113(17–18):676–80.
Mongardon N, Legriel S, Lemiale V, Cariou A. Prediction of neurological outcome after cardiac arrest: is serum procalcitonin the future? Neurocrit Care. 2010;13(1):159–60.
Mongardon N, Lemiale V, Perbet S, Dumas F, Legriel S, Guerin S, et al. Value of procalcitonin for diagnosis of early onset pneumonia in hypothermia-treated cardiac arrest patients. Intensive Care Med. 2010;36(1):92–9.
Albrich WC, Dusemund F, Bucher B, Meyer S, Thomann R, Kuhn F, et al. Effectiveness and safety of procalcitonin-guided antibiotic therapy in lower respiratory tract infections in “real life”: an international, multicenter poststudy survey (ProREAL). Arch Intern Med. 2012;172(9):715–22.
Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–74.
Burkhardt O, Ewig S, Haagen U, Giersdorf S, Hartmann O, Wegscheider K, et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. Eur Respir J. 2010;36(3):601–7.
Christ-Crain M, Stolz D, Bingisser R, Muller C, Miedinger D, Huber PR, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84–93.
Schuetz P, Briel M, Mueller B. Clinical outcomes associated with procalcitonin algorithms to guide antibiotic therapy in respiratory tract infections. JAMA. 2013;309(7):717–8.
Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322–31.
Bafadhel M, Clark TW, Reid C, Medina MJ, Batham S, Barer MR, et al. Procalcitonin and C-reactive protein in hospitalized adult patients with community-acquired pneumonia or exacerbation of asthma or COPD. Chest. 2011;139(6):1410–8.
Muller B, Harbarth S, Stolz D, Bingisser R, Mueller C, Leuppi J, et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect Dis. 2007;7:10.
Bloos F, Marshall JC, Dellinger RP, Vincent JL, Gutierrez G, Rivers E, et al. Multinational, observational study of procalcitonin in ICU patients with pneumonia requiring mechanical ventilation: a multicenter observational study. Crit Care. 2011;15(2):R88.
Duflo F, Debon R, Monneret G, Bienvenu J, Chassard D, Allaouchiche B. Alveolar and serum procalcitonin: diagnostic and prognostic value in ventilator-associated pneumonia. Anesthesiology. 2002;96(1):74–9.
Luyt CE, Combes A, Reynaud C, Hekimian G, Nieszkowska A, Tonnellier M, et al. Usefulness of procalcitonin for the diagnosis of ventilator-associated pneumonia. Intensive Care Med. 2008;34(8):1434–40.
Ramirez P, Garcia MA, Ferrer M, Aznar J, Valencia M, Sahuquillo JM, et al. Sequential measurements of procalcitonin levels in diagnosing ventilator-associated pneumonia. Eur Respir J. 2008;31(2):356–62.
Acknowledgements
The study was supported by the Centre Hospitalier de Versailles, Versailles, France. We thank the nurses in our medical-surgical intensive care unit for their contribution to the study, M. Bouery-Veysseyre for logistic help, and A. Wolfe for help in preparing the manuscript.
Funding
The study was supported by the Centre Hospitalier de Versailles, Versailles, France.
Author information
Authors and Affiliations
Consortia
Contributions
SL and JPB conceived, designed, and supervised the trial. All the investigators collected the data. SL and BG coordinated the data collection. PM was in charge of the statistical analysis. SL and BG analyzed and interpreted the data. MB and AM performed PCT assays. SL and BG wrote the first draft of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Legriel, S., Grigoresco, B., Martel, P. et al. Diagnostic Accuracy of Procalcitonin for Early Aspiration Pneumonia in Critically Ill Patients with Coma: A Prospective Study. Neurocrit Care 30, 440–448 (2019). https://doi.org/10.1007/s12028-018-0623-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-018-0623-8