Abstract
Background
To determine the cerebral protective effects of mild hypothermia (MH) on cerebral microcirculation.
Methods
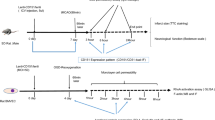
We established ischemia–reperfusion (I/R) injury and MH treatment models with rat brain microvascular endothelial cells (RBMECs) in vitro and examined the apoptotic changes. The cultured RBMECs were randomly divided into the control group, I/R group, and MH group, which was further divided into two subgroups: intra-ischemia hypothermia (IIH) and post-ischemia hypothermia (PIH). Cell morphological changes were assessed using fluorescence microscopy. Apoptotic rates were obtained by flow cytometry. Expressions of caspase-3, Bax, and Bcl-2 were analyzed by Western blot.
Results
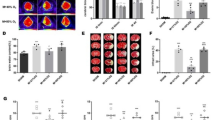
I/R injury in vitro induced apoptosis of RBMECs. The apoptotic rates in the control group, I/R group, and MH group were 0.13, 19.04, and 13.13%, respectively (P < 0.01). Compared with the I/R group, the MH group showed a significant decrease in the number of apoptotic cells, mainly in stage I apoptotic cells (P < 0.0083). The caspase-3 and Bax expressions were significantly enhanced (P < 0.05) in RBMECs after I/R injury, while substantial decreases in Bcl-2 expression were noted (P < 0.05). Following MH intervention, the increase in caspase-3 and Bax expression was suppressed (P < 0.05), while Bcl-2 expression significantly increased. The apoptotic rates or protein expressions between the two subgroups were not different significantly (P > 0.05).
Conclusions
These results indicate that MH could inhibit RBMEC apoptosis by preventing pro-apoptotic cells and early apoptotic cells from progressing to intermediate and advanced stages. This may be due to the effect of MH on I/R-induced apoptotic gene expression changes.




Similar content being viewed by others
References
Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Bbttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T, International Liaison Committee on R, Emergency Cardiovascular Care Committee AHA, Council on Cardiovascular S, Anesthesia, Council on Cardiopulmonary P, Critical C, Council on Clinical C, Council on S. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke (Part II). Int Emerg Nurs. 2010;18(1):8–28. https://doi.org/10.1016/j.ienj.2009.07.001.
Wong CH, Bozinovski S, Hertzog PJ, Hickey MJ, Crack PJ. Absence of glutathione peroxidase-1 exacerbates cerebral ischemia–reperfusion injury by reducing post-ischemic microvascular perfusion. J Neurochem. 2008;107(1):241–52. https://doi.org/10.1111/j.1471-4159.2008.05605.x.
Ristagno G, Tang W, Sun S, Weil MH. Cerebral cortical microvascular flow during and following cardiopulmonary resuscitation after short duration of cardiac arrest. Resuscitation. 2008;77(2):229–34. https://doi.org/10.1016/j.resuscitation.2007.12.013.
Teschendorf P, Padosch SA, Del Valle YFD, Peter C, Fuchs A, Popp E, Spohr F, Bottiger BW, Walther A. Effects of activated protein C on post cardiac arrest microcirculation: an in vivo microscopy study. Resuscitation. 2009;80(8):940–5. https://doi.org/10.1016/j.resuscitation.2009.04.029.
Fink K, Schwarz M, Feldbrugge L, Sunkomat JN, Schwab T, Bourgeois N, Olschewski M, von Zur Muhlen C, Bode C, Busch HJ. Severe endothelial injury and subsequent repair in patients after successful cardiopulmonary resuscitation. Crit Care. 2010;14(3):R104. https://doi.org/10.1186/cc9050.
Huet O, Dupic L, Batteux F, Matar C, Conti M, Chereau C, Lemiale V, Harrois A, Mira JP, Vicaut E, Cariou A, Duranteau J. Postresuscitation syndrome: potential role of hydroxyl radical-induced endothelial cell damage. Crit Care Med. 2011;39(7):1712–20. https://doi.org/10.1097/CCM.0b013e3182186d42.
Grundmann S, Fink K, Rabadzhieva L, Bourgeois N, Schwab T, Moser M, Bode C, Busch HJ. Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation. 2012;83(6):715–20. https://doi.org/10.1016/j.resuscitation.2012.01.028.
Smrcka M, Horky M, Otevrel F, Kuchtickova S, Kotala V, Muzik J. The onset of apoptosis of neurons induced by ischemia–reperfusion injury is delayed by transient period of hypertension in rats. Physiol Res. 2003;52(1):117–22.
Armstrong SC. Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61(3):427–36. https://doi.org/10.1016/j.cardiores.2003.09.031.
Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56. https://doi.org/10.1056/NEJMoa012689.
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63. https://doi.org/10.1056/NEJMoa003289.
Janata A, Holzer M. Hypothermia after cardiac arrest. Prog Cardiovasc Dis. 2009;52(2):168–79. https://doi.org/10.1016/j.pcad.2009.07.001.
Elmer J, Polderman KH. Emergency neurological life support: resuscitation following cardiac arrest. Neurocrit Care. 2017;. https://doi.org/10.1007/s12028-017-0457-9.
Xu L, Yenari MA, Steinberg GK, Giffard RG. Mild hypothermia reduces apoptosis of mouse neurons in vitro early in the cascade. J Cereb Blood Flow Metab. 2002;22(1):21–8. https://doi.org/10.1097/00004647-200201000-00003.
Liu L, Kim JY, Koike MA, Yoon YJ, Tang XN, Ma H, Lee H, Steinberg GK, Lee JE, Yenari MA. FasL shedding is reduced by hypothermia in experimental stroke. J Neurochem. 2008;106(2):541–50. https://doi.org/10.1111/j.1471-4159.2008.05411.x.
Li H, Wang D. Mild hypothermia improves ischemic brain function via attenuating neuronal apoptosis. Brain Res. 2011;1368:59–64. https://doi.org/10.1016/j.brainres.2010.10.073.
Luo C, Pan SY. The pathways by which mild hypothermia inhibits neuronal apoptosis following ischemia/reperfusion injury. Neural Regen Res. 2015;10(1):153–8. https://doi.org/10.4103/1673-5374.150725.
Gong P, Zhao S, Wang J, Yang Z, Qian J, Wu X, Cahoon J, Tang W. Mild hypothermia preserves cerebral cortex microcirculation after resuscitation in a rat model of cardiac arrest. Resuscitation. 2015;97:109–14. https://doi.org/10.1016/j.resuscitation.2015.10.003.
Wu Z, Hofman FM, Zlokovic BV. A simple method for isolation and characterization of mouse brain microvascular endothelial cells. J Neurosci Methods. 2003;130(1):53–63.
Calabria AR, Weidenfeller C, Jones AR, de Vries HE, Shusta EV. Puromycin-purified rat brain microvascular endothelial cell cultures exhibit improved barrier properties in response to glucocorticoid induction. J Neurochem. 2006;97(4):922–33. https://doi.org/10.1111/j.1471-4159.2006.03793.x.
Kim JA, Tran ND, Li Z, Yang F, Zhou W, Fisher MJ. Brain endothelial hemostasis regulation by pericytes. J Cereb Blood Flow Metab. 2006;26(2):209–17. https://doi.org/10.1038/sj.jcbfm.9600181.
Schlosshauer B. The blood-brain barrier: morphology, molecules, and neurothelin. BioEssays News Rev Mol Cell Dev Biol. 1993;15(5):341–6. https://doi.org/10.1002/bies.950150508.
Xie Y, Ye LY, Zhang XB, Hou XP, Lou JN. Establishment of an in vitro model of brain-blood barrier. Beijing da xue xue bao Yi xue ban = J Peking Univ Health Sci. 2004;36(4):435–8.
Hiu T, Nakagawa S, Hayashi K, Kitagawa N, Tsutsumi K, Kawakubo J, Honda M, Suyama K, Nagata I, Niwa M. Tissue plasminogen activator enhances the hypoxia/reoxygenation-induced impairment of the blood-brain barrier in a primary culture of rat brain endothelial cells. Cell Mol Neurobiol. 2008;28(8):1139–46. https://doi.org/10.1007/s10571-008-9294-x.
Bernas MJ, Cardoso FL, Daley SK, Weinand ME, Campos AR, Ferreira AJ, Hoying JB, Witte MH, Brites D, Persidsky Y, Ramirez SH, Brito MA. Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier. Nat Protoc. 2010;5(7):1265–72. https://doi.org/10.1038/nprot.2010.76.
Pozarowski P, Grabarek J, Darzynkiewicz Z (2003) Flow cytometry of apoptosis. Current protocols in cytometry Chapter 7: Unit 7 19. https://doi.org/10.1002/0471142956.cy0719s25.
Telford W, Tamul K, Bradford J. Measurement and characterization of apoptosis by flow cytometry. Curr Protoc Cytom. 2016;77:1–9. https://doi.org/10.1002/cpcy.1.
Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4(5):399–415. https://doi.org/10.1038/nrn1106.
Matsui T, Yoshida Y, Yanagihara M, Suenaga H. Hypothermia at 35 °C reduces the time-dependent microglial production of pro-inflammatory and anti-inflammatory factors that mediate neuronal cell death. Neurocrit Care. 2014;20(2):301–10. https://doi.org/10.1007/s12028-013-9911-5.
Antonova OA, Loktionova SA, Romanov YA, Shustova ON, Khachikian MV, Mazurov AV. Activation and damage of endothelial cells upon hypoxia/reoxygenation. Effect of extracellular pH. Biochem Biokhimiia. 2009;74(6):605–12.
Yang D, Guo S, Zhang T, Li H. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Lett. 2009;583(15):2500–6. https://doi.org/10.1016/j.febslet.2009.07.006.
Zhang Y, Zhang X, Park TS, Gidday JM. Cerebral endothelial cell apoptosis after ischemia–reperfusion: role of PARP activation and AIF translocation. J Cereb Blood Flow Metab. 2005;25(7):868–77. https://doi.org/10.1038/sj.jcbfm.9600081.
Miclescu A, Sharma HS, Martijn C, Wiklund L. Methylene blue protects the cortical blood-brain barrier against ischemia/reperfusion-induced disruptions. Crit Care Med. 2010;38(11):2199–206. https://doi.org/10.1097/CCM.0b013e3181f26b0c.
Li YQ, Chen P, Haimovitz-Friedman A, Reilly RM, Wong CS. Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Can Res. 2003;63(18):5950–6.
Kinnally KW, Antonsson B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis Int J Program Cell Death. 2007;12(5):857–68. https://doi.org/10.1007/s10495-007-0722-z.
Xi HJ, Zhang TH, Tao T, Song CY, Lu SJ, Cui XG, Yue ZY. Propofol improved neurobehavioral outcome of cerebral ischemia–reperfusion rats by regulating Bcl-2 and Bax expression. Brain Res. 2011;1410:24–32. https://doi.org/10.1016/j.brainres.2011.06.060.
Nie C, Dong J, Zhang P, Liu X, Han F. Prehospital therapeutic hypothermia after out-of-hospital cardiac arrest: a systematic review and meta-analysis. Am J Emerg Med. 2016;34(11):2209–16. https://doi.org/10.1016/j.ajem.2016.09.007.
Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109(22):2786–91. https://doi.org/10.1161/01.CIR.0000131940.19833.85.
Nozari A, Safar P, Stezoski SW, Wu X, Kostelnik S, Radovsky A, Tisherman S, Kochanek PM. Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation. 2006;113(23):2690–6. https://doi.org/10.1161/CIRCULATIONAHA.106.613349.
Zhao D, Abella BS, Beiser DG, Alvarado JP, Wang H, Hamann KJ, Hoek TL, Becker LB. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation. 2008;77(2):242–9. https://doi.org/10.1016/j.resuscitation.2007.10.015.
Wang H, Barbut D, Tsai MS, Sun S, Weil MH, Tang W. Intra-arrest selective brain cooling improves success of resuscitation in a porcine model of prolonged cardiac arrest. Resuscitation. 2010;81(5):617–21. https://doi.org/10.1016/j.resuscitation.2010.01.027.
Darbera L, Chenoune M, Lidouren F, Kohlhauer M, Adam C, Bruneval P, Ghaleh B, Dubois-Rande JL, Carli P, Vivien B, Ricard JD, Berdeaux A, Tissier R. Hypothermic liquid ventilation prevents early hemodynamic dysfunction and cardiovascular mortality after coronary artery occlusion complicated by cardiac arrest in rabbits. Crit Care Med. 2013;41(12):e457–65. https://doi.org/10.1097/CCM.0b013e3182a63b5d.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC: 81201446, https://isis.nsfc.gov.cn). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Wang, L., Zhang, Y. et al. The Effect of Therapeutic Mild Hypothermia on Brain Microvascular Endothelial Cells During Ischemia–Reperfusion Injury. Neurocrit Care 28, 379–387 (2018). https://doi.org/10.1007/s12028-017-0486-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-017-0486-4