Abstract
TSH receptor (TSHR) autoantibody (TRAb) is the serological hallmark of Graves’ disease (GD). Third-generation enzyme-linked immunosorbent assays (ELISAs) using monoclonal TRAbs instead of TSH have been found useful for TRAb analysis recently. For the first time, a mouse monoclonal antibody (mAb) against TSHR was analyzed for TRAb detection and compared with human mAb M22 and TSH by the same competitive binding assay technique. A mouse monoclonal antibody (T7) binding to the TSH receptor and inhibiting TSH binding was generated and used for TRAb analysis in a third-generation ELISA. Obtained TRAb levels were compared with a second-generation TRAb assay employing bovine TSH and a third-generation assay with human mAb M22 as TSHR-binding reagents by investigating 89 patients with GD, 56 with Hashimoto’s thyroiditis (HT), 73 with non-autoimmune thyroid diseases, 17 with rheumatoid arthritis, and 100 healthy subjects. The T7-based TRAb ELISA did not reveal a significantly different assay performance (area under the curve [AUC]) in contrast to the TSH and M22-based TRAb ELISAs by receiver operating characteristic (ROC) curve analysis (AUC-T7 0.967, AUC-TSH 0.972, AUC-M22 0.958, p > 0.05, respectively). After adjustment of cutoffs by ROC, all three TRAb ELISAs demonstrated sensitivities and specificities above 89.9% and 96.0%, respectively. Both third-generation TRAb ELISAs showed a tendency for a higher prevalence of TRAb positives in HT in contrast to the second-generation ELISA. Mouse mAbs against the TSHR may be used for the reliable detection of TRAb by third-generation TRAb ELISA. The earlier reported higher sensitivity of third-generation TRAb ELISA in GD needs to be considered in the context of a slightly lower specificity regarding HT.

Similar content being viewed by others
Introduction
Three generations of competitive binding TSH receptor (TSHR) autoantibody (TRAb) tests employing different TSHR-binding ligands for the detection of TSHR-binding inhibitory immunoglobulin (TBII) have been used in the serological diagnosis of Graves’ disease (GD) [1, 2]. Through their binding to the TSHR, TRAb can exert a stimulatory signal on thyrocytes and, thus, have a leading pathogenic role in GD development. However, such so-called TSHR stimulatory antibodies (TSAb) can only be detected by cell-based bioassays and discriminated from TSHR blocking ones (TBAb) [3]. TSHR-binding competitive and bridge assay reaction environments do not appear to do so and, for the sake of clarity, TBII will be referred to as TRAb below. TSAb and TBAb appear to share common overlapping epitopes of the TSH-binding site which hinders them to be distinguished by competitive and bridge TRAb assays [3,4,5].
Competitive binding TRAb assays depend on TRAb inhibition of labeled TSH binding to TSHR preparations in fluid phase (first generation) or immobilized TSHR on solid phases (second generation) [6,7,8,9]. Third-generation competitive TRAb assays use immobilized TSHR for bound/free separation and monoclonal antibodies (mAbs) like the human stimulatory moAb M22 or recombinant mouse mAb mimicking the binding of TSH to the TSHR for the competitive assay environment [10, 11]. There has been a continuous improvement of assay performance from test generation to test generation [12]. Thus, the high affinity of M22 being the first mAb used for TRAb analysis by ELISA, its slow dissociation rate, and intrinsic stability rendered it a particularly suitable TSHR ligand, paving the way for the third-generation of competitive binding TRAb assays [13].
We generated the mouse mAb T7 which interacts with the TSH-binding site on the TSHR to investigate whether mouse mAb can be used for TRAb analysis, too. Thus, we developed and evaluated a third-generation TRAb competitive detection environment by using (i) a mAb for TSHR immobilization on an ELISA solid phase not interfering with the TSH-TSHR interaction and (ii) the TSHR-binding mAb T7 inhibiting TRAb binding to the TSHR.
Patients and methods
Serum samples of 218 adult patients attending the thyroid unit of the Department of Nuclear Medicine of the University Hospital Carl Gustav Carus, TU Dresden, were recruited for the study (Table 1). The patients suffered from Graves’ disease (GD, n = 89), Hashimoto’s thyroiditis (HT, n = 56), and non-autoimmune thyroid disease (NAITD, n = 70). Age, sex, thyroglobulin antibodies (TgAb), thyroid peroxidase antibodies (TPOAb), TSH, and thyroid hormone levels are shown in Table 1. Furthermore, 16 rheumatoid factor-positive patients with rheumatoid arthritis (RA) and 100 healthy subjects (in.vent, Hennigsdorf, Berlin) were included as controls into the study.
GD was diagnosed on the basis of clinical symptoms, biochemical confirmation of hyperthyroidism, and additional diagnostic information, such as goiter, ophthalmopathy, thyroid ultrasound, and thyroid scintigraphy. The patients were at various stages of their diseases (e.g., some were on thyroid medication, had radioiodine treatment, or thyroidectomy previously). The time interval between initial diagnosis and TRAb determination was high and ranged from 1 month to 3.5 years.
We established a patient cohort with well-defined HT by recruiting patients suspected of suffering from autoimmune thyroid disease with repeated examinations. A history of transient and often only slightly intense thyrotoxicosis followed by gradually developing hypothyroidism, high titers of TPOAb and/or TgAb, hypoechoic lesions on ultrasound, and lymphocytic infiltrates on fine needle aspiration cytology were considered to support the diagnosis of HT. This diagnosis was then reviewed at every follow-up to reach a very high probability for HT. Of note, a small uncertainty for misclassification still remains, particularly in the population with borderline or low-positive TRAb we examined here. Most of these patients were on thyroid hormone medication, but no one had a long period of anti-thyroid drug medication or a definitive treatment.
Non-autoimmune thyroid disease (NAITD) was diagnosed on the basis of clinical symptoms, biochemical confirmation of hyperthyroidism, and additional diagnostic results, such as diffuse and/or nodular goiter, thyroid ultrasound, and thyroid scintigraphy.
Third-generation TRAb assays based on a murine monoclonal antibody
Mouse mAb against the TSHR were generated by immunization of mice with human TSHR expressed on cells similarly to an approach described elsewhere to develop a third-generation TRAb ELISA (Medizym T.R.A. human, Medipan GmbH, Germany) [14]. Briefly, a mouse mAb binding to the TSHR and not interfering with TSH binding was selected and coated on the surface of polystyrene 96-well plates (MaxiSorp, Thermo Fisher Scientific GmbH, Germany) to immobilize human TSHR. Neat patient serum or calibrators were incubated for 2 h at room temperature (RT) while shaking. After another wash cycle (three times for 1 min each), biotinylated mAb T7 was added and incubated at RT for 2 h while shaking. Specific binding of biotinylated T7 to the remaining immobilized TSHR not bound by TRAb was revealed by incubating streptavidin-poly-horseradish peroxidase (Thermo Fisher Scientific GmbH) and 3,3′,5,5-tetramethylbezidine (Seramun Diagnostica GmbH, Germany) consecutively. After stopping the enzymatic turnover of the substrate by sulfuric acid, optical densities were measured by a photometer at 450 nm against 620 nm and used for TRAb detection by a software program.
In the first instance, a cutoff of 1.5 international units (IU)/L was established. Inter-assay coefficients of variation (CV) were determined in accordance with CLSI protocol EP15-A2 using 3 different lots at 3.7% and 6.0% for sera with TRAb values of 0.9 IU/L and 14.3 IU/L, respectively.
Established second- and third-generation TRAb assays
Two commercially available assays were used for the comparison with the novel third-generation TRAb ELISA. A second-generation TRAb (Medizym T.R.A., Medipan GmbH, Germany) based on the ability of TRAb to inhibit the binding of biotinylated bovine TSH to porcine TSHR was employed. Following the manufacturer’s recommendations, TRAb values below 1.5 IU/L were defined as negative in this TRAb assay. Further, a third-generation TRAb ELISA (Medizym TRAb clone, Medipan GmbH, Germany) based on the ability of TRAb to inhibit the binding of the biotinylated human mAb M22 to TSHR of porcine origin was used as described elsewhere [15]. M22-biotin binding to the TSHR immobilized on the solid phase was detected by addition of streptavidin peroxidase. The manufacturers’ recommended cutoff limit for the M22-based TRAb ELISA is 0.4 IU/L. Both assays were calibrated against WHO standard NIBSC 90/672.
The analytical sensitivities (lower detection limit), the inter-assay variations, and functional assay sensitivities (fas) for both assays were determined previously [15,16,17]. Thus, the Medizym TRA and the Medizym TRAb clone have fas of 0.9 and 0.3 IU/L, respectively.
Statistical analysis
Statistical analysis was performed using the MedCalc® program (MedCalc software, Belgium). The two-tailed, Mann-Whitney and Kruskal–Wallis tests were used to test for statistically significant differences of independent samples in 2 and more groups, respectively. Prevalence comparison between groups was performed by two-tailed Fisher’s exact test. Method comparison was performed by the Passing-Bablok regression model which is a linear regression procedure with no special assumptions regarding the distribution of the samples and the measurement errors. The result of the analysis does not depend on the assignment of the TRAb values to X and Y. The slope and intercept are calculated with their 95% confidence interval (CI). Further, assay performance data like specificity, sensitivity, and positive and negative likelihood ratios as well as area under the curve (AUC) were determined by receiver operating characteristic (ROC) curve analysis. Significance was defined as p < 0.05.
Results
Demographic and thyroid laboratory characteristics of patients and controls
Patients with GD (n = 89) were compared with an age- and sex-matched group of 246 disease controls and HS (Table 1). However, in terms of comparison with single control cohorts, there was a significant difference of age and sex of GD patients with NAITD patients (p < 0.05, respectively).
In terms of thyroid hormone levels, patients with HT demonstrated a significantly higher TSH level and both significantly lower fT3 as well as fT4 levels compared with GD patients (p < 0.05, respectively).
Regarding autoantibody levels, GD patients and HT patients showed both significantly higher TPOAb and TgAb levels in contrast to NAITD patients (p < 0.05, respectively).
Functional assay sensitivity of the T7-based TRAb ELISA
The fas of the mouse mAb T7-based TRAb ELISA was determined by inter-assay CV of eight determinations for eight TRAb-positive sera on five different days in accordance with the CLSI protocol EP15-A2. A value of 0.9 IU/L was determined as fas. The assay was calibrated against the WHO international standard for thyroid-stimulating antibody (NIBSC code 08/204).
Regression analysis of the T7-based with second- and third-generation TRAb assays
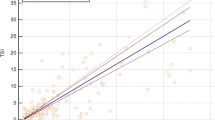
All 335 sera were run in the mouse mAb T7-based TRAb assay and obtained TRAb levels were compared with those of the bovine TSH-based (second generation) as well as of the human mAb M22-based TRAb (third generation) assays by Passing-Bablok regression analysis and residual plots (Fig. 1). The comparison of the T7-based TRAb ELISA with the two latter TRAb assays revealed the following regression equations: y = 0.01 + 0.99 x and y = − 1.83 + 2.24 x, respectively (Fig. 2). For both regression analyses, there is a significant deviation from linearity by CUSUM test (p < 0.01, respectively). The residual plots demonstrate a tendency for lower TRAb values detected by the T7-based TRAb third-generation ELISA. However, there appears to be no proportional difference as well as no difference by a constant amount between the T7-based third-generation and the TSH-based second-generation TRAb ELISA since the slope 95% CI contains the value 1.00 (95% CI 0.89–1.11) and the value 0 was covered by the intercept 95% CI (− 0.05–0.07). In contrast, the comparison of both third-generation TRAb ELISAs (T7-based with the M22-based TRAb assay) showed a proportional difference (slope 95% CI 1.96–2.70) as well as a difference by a constant amount (intercept 95% CI − 0.33 to − 0.08). Noteworthy, the TSH-based and M22-based TRAb ELISA differ by a constant amount and a proportional difference, too (y = 0.07 + 0.51 x, intercept 95% CI 0.05–0.10, slope 95% CI 0.47–0.54).
Comparison of TRAb levels determined by second- and third-generation (TRAb) ELSIA using different TSH receptor-binding molecules for competing with TRAb in 89 patients with Graves’ disease (GD), 56 with Hashimoto’s thyroiditis (HT), 73 with non-autoimmune thyroid diseases (NAITD), 17 with rheumatoid arthritis (RA), and 100 healthy subjects (HS). The diagonal line demonstrates the line of equality. The vertical and horizontal lines represent recommended and adjusted cutoff values of the T7-based (1.5 IU/L, 2.0 IU/l), M22-based (0.4 IU/l, 0.8 IU/L), and TSH-based TRAb ELISA (1.5 IU/L, 1.4 IU/L), respectively
Comparison of qualitative TRAb results in patients with GD and controls
Qualitative TRAb results using the recommended cutoff for each TRAb ELISA are shown in Table 2. The comparison of all 335 patients and controls obtained for the T7-based TRAb ELISA with those of the TSH-based and M22-based TRAb ELISA revealed significant differences (4.9%, 95% CI 2.2–5.6%, p = 0.0005; 18.2%, 95% CI 14.2–20.3%, p < 0.0001, respectively). Whereas there were no significant differences for the comparison of the T7-based with the TSH-based TRAb ELISA in accordance with the McNemar test regarding the various cohorts, HT patients revealed a significant difference in terms of the comparison of both third-generation TRAb ELISAs (T7-based vs. M22-based TRAb ELISA, Table 3). This was due to the significantly higher number of positives by the M22-based TRAb ELISA in contrast to the T7-based one (28/56 vs. 11/56, p = 0.0013).
Thus, the M22-based TRAb ELISA demonstrated the highest sensitivity with 94.4% vs. 91.0% and 93.3% (TSH-based and T7-based TRAb ELISA), respectively, of all three TRAb ELISA but was rather unspecific (85.0% vs. 97.6% and 94.3%, respectively).
Receiver operating characteristic curve analysis
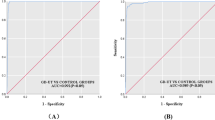
To compare all three TRAb ELISA on a quantitative level, ROC curve analysis was performed (Fig. 3). The AUC of the T7-based TRAb ELISA was not significantly different from the AUCs of the other two TRAb ELISAs. Noteworthy, the TSH-based TRAb ELISA demonstrated the highest AUC which was not significantly higher than the lowest AUC determined for the M22-based TRAb ELISA. In accordance with the ROC curve analysis of the patient and control cohorts of this study, the following optimized cutoff values were determined for the TSH-, M22-, and T7-based TRAb assays: 1.4 IU/L, 0.8 IU/L, and 2.0 IU/L, respectively.
Comparison of receiver operating characteristic (ROC) curve analysis of second- and third-generation TRAb ELISA using the TRAb levels of 89 patients with Graves’ disease (GD) as disease variable and TRAb levels of 56 with Hashimoto’s thyroiditis (HT), 73 with non-autoimmune thyroid diseases (NAITD), 17 with rheumatoid arthritis (RA) as well as 100 healthy subjects (HS) as control variable. The following areas under the curve (AUC) were obtained: T7-based third-generation TRAb, AUC = 0.967; 95% CI 0.949–0.987. M22-based third-generation TRAb, AUC = 0.958; 95% CI 0.931–0.977. TSH-based second-generation TRAb, AUC = 0.972; 95% CI 0.949–0.987. The AUC differences between the three TRAb ELISAs were not significant (p > 0.05)
Comparison of qualitative TRAb results in patients with GD and controls after cutoff adjustment
Based on the optimized cutoff values, TRAb positives were determined in the patients and HS cohorts (Table 2, Fig. 2). Thus, the T7-based TRAb ELISA demonstrated the highest sensitivity (92.1% vs. 89.9% and 91.0%; TSH-based and M22-based TRAb ELISAs respectively) of all three TRAb ELISA with a corresponding specificity of 96.3%. The comparison of all qualitative 335 patient’s and control’s TRAb data of the three TRAb ELISAs with the optimized cutoff values did not reveal significant differences between the methods (p > 0.05, respectively). Accordingly, no significant differences were determined in the single patient and control cohorts.
Please note that 79 out of 89 GD patients (88.8%) scored positive and 6 (6.7%) negative in all three TRAb assays after adjustment of cutoffs. Thus, all three TRAb ELISA demonstrated an agreement higher than 95% within GD patients. The six GD patients with negative results in all three TRAb ELISAs had normal TSH and thyroid hormone levels and were positive for either TPOAb or TgAb or both. Likewise, the two single TRAb-positive GD patients detected by either of the third-generation TRAb ELISA showed normal TSH and thyroid hormone levels and were positive for either TPOAb or TgAb or both, too.
In terms of disease controls, three HT patients demonstrated positive TRAb levels by all three TRAb ELISA after ROC-based adjustment of the cutoff values. Two of them showed reduced TSH levels below 0.3 mU/L, whereas the remaining HT patient had an elevated TSH level (9.9 mU/L). All three patients were either positive for TPOAb or TgAb or both, received thyroid hormone replacement therapy, and had euthyroid hormone levels.
One out of the five single-positive HT patients detected by the T7-based TRAb ELISA had a slightly elevated TSH level (5.4 mU/L) with normal thyroid hormones. The remaining four patients showed normal TSH and hormone levels.
Discussion
The generation of mAbs directed against the TSH receptor which could be used for the immobilization thereof onto solid phases without interfering with the TSH binding and mAbs inhibiting TSH binding ushered in the era of competitive binding third-generation TRAb assays [2, 6, 11, 18,19,20]. The thyroid-stimulating human mAb M22 derived from peripheral blood mononuclear cells of a GD patient was employed to set up the first third-generation TRAb ELISA [15, 21].
Recently, a non-competitive TRAb bridge assay was reported which demonstrated similar assay performance like competitive binding assay variants [11]. Notably, both competitive and non-competitive TRAb assays do not differentiate TBAb from TSAb [22,23,24]. However, TSHR bioassays, the sole assay variants enabling TSAb/TBAb differentiation, have not been introduced into routine laboratories widely yet, although they have proven practical usefulness despite their challenging assay design [25, 26].
We generated the mouse mAb T7 inhibiting TSH binding to human TSHR in accordance with a methodology described elsewhere [27]. The novel T7-based third-generation TRAb ELISA has a fas of 0.9 IU/L which is below the recommended cutoff of 1.5 IU/L. Hence, this TRAb ELISA can be used for the serological discrimination of TRAb-positive from TRAb-negative patients.
Comparison of the novel TRAb ELISA with the first third-generation TRAb assay based on the human mAb M22 and a second-generation TRAb ELISA revealed no statistical differences with regard to assay performance analyzed by ROC curve analysis. Thus, all three assay designs may be used for the detection of TRAb aiding in the serological diagnosis of GD. To the best of our knowledge, this is the first comparison of second- and third-generation TRAb assays by employing the same assay technique. Hence, it may be less influenced by assay characteristics like labelling methods and readout variants. Given the similar assay performance of all three TRAb assays investigated, the use of different sources of TSHR did not appear to have an effect on the comparability of TRAb assays. This finding supports earlier studies which could not determine differences in the TRAb assay performance comparing for instance human and porcine TSHR preparations for TRAb analysis [16].
Passing-Bablok regression analysis and Bland-Altman plots revealed a good correlation of mean TRAb values up to 10 IU/L. Mean TRAb values above 10 IU/L tend to be determined higher in the TSH-based second-generation in contrast to the third-generation TRAb ELISA. Both third-generation TRAb ELISAs did not reveal a clear tendency in that respect that might have an impact on treatment choices in GD [28], since higher TRAb levels are associated with a higher risk of relapse in GD patients [29, 30].
Applying the cutoff values recommended by the manufacturer, the M22-based third-generation TRAb assay demonstrated the highest sensitivity (94.4%) in contrast to the other TRAb assays (91.0% for the TSH-based and 93.3% for the T7-based) of this study but a lower specificity of 85.0%. This finding is in line with previous reports of a higher sensitivity of third-generation in contrast to second-generation TRAb assays. However, the lower specificity might be due to the higher number of patients with HT and NAITD recruited in this study in contrast to previous ones [17, 31]. The HT cohort described here revealed 28/56 (50.0%) positives for the M22-based third-generation TRAb ELISA compared to the previously reported frequency of 9/26 (34.6%) [17]. Previous studies indicated that about 10% of patients with HT might demonstrate TRAb detectable by second-generation TRAb assays [6, 32, 33]. For the latter assay technique, this value was reproduced in this study, whereas the novel T7-based third-generation TRAb ELISA demonstrated a twice higher positivity rate in this patient cohort. Notably, even TSAb could be detected by a bioassay in HT patients particularly in those with ophthalmopathy [34]. However, none of our patients with HT scoring positive in the third-generation TRAb ELISA was diagnosed with ophthalmopathy. Altogether, the novel T7-based third-generation as well as the TSH-based second-generation TRAb ELISA revealed specificities well beyond 90% for all control cohorts.
Of note, the ROC curve analysis performed in this study revealed different cutoff values in contrast to the ones obtained by earlier assay performance analyses. In particular, the adapted cutoff values of the third-generation TRAb assays deviated from the recommended ones (M22-based TRAb ELISA, 0.8 IU/L vs. 0.4 IU/L; T7-based TRAb ELISA, 2.0 IU/L vs. 1.5 IU/L). As a fact, the qualitative discrepancies between the three TRAb ELISAs were reduced after adjustment of all three cutoff values in accordance with the ROC curve analysis. Both third-generation TRAb ELISAs benefited from their respective higher cutoff values, which diminished the number of positives in the control groups. Further, the adjusted cutoff of 0.8 IU/L for the M22-based third-generation TRAb ELISA is well discriminated from the previously reported fas of 0.3 IU/L which improves its precision [15]. A somewhat poorer imprecision was recently reported for the M22-based TRAb ELISA by Liu et al. [35]. The authors determined a fas of 1.8 IU/L; however, they used a different assay design with shorter incubation times than originally reported in 2004 [15].
After adjustment of the cutoff values, the T7-based third-generation TRAb ELISA demonstrated the highest sensitivity of 92.1% but a lower specificity of 96.3% compared with the other two TRAb ELISAs. First of all, this was due to the higher number of positive HT patients (8/56, 14.3%) in this TRAb ELISA which was slightly higher than the 10% in average reported elsewhere [3, 7]. In contrast, a recently published third-generation TRAb fluoroenzyme assay employing a mouse recombinant mAb did not detect elevated TRAb in HT, whereas a radioimmunometric as well as a bridge TRAb assay did in that study [11].
Notably, 3 HT patients showed elevated TRAb levels in all three ELISA of our study, whereas two of them demonstrated TSH levels below 0.3 mU/L while having thyroid hormone replacement therapy. Altogether, all three TRAb ELISAs revealed acceptable specificities beyond 96.0%. Furthermore, all three TRAb ELISAs were not affected by rheumatoid factor positivity which underscores the satisfactory specificity of the competitive binding TRAb ELISA reaction environment.
A certain limitation of the present study might be the recruitment of patients whose diagnosis was established by using routinely determined TRAb levels of a bovine TSH-based second-generation TRAb radioimmunoassay. It may be that some sera from potential GD patients with TRAb values below 0.6 IU/L in the TRAb radioimmunoassay were positive in the second- or third-generation TRAb ELISAs. As a result, these patients would not have been included in our patient population. Moreover, samples from HT or NAITD patients with higher TRAb levels in the TRAb radioimmunoassay might have been scored negative in the TRAb ELISA and would not have been included in our patient cohorts either.
In summary, the present second- and third-generation TRAb ELISA demonstrated a comparable assay performance in terms of the serological diagnosis of GD. Mouse mAbs may be used instead of human ones like the M22 for the reliable detection of TRAb in daily clinical routine. The higher sensitivity of third-generation TRAb ELISA in GD needs to be considered in the context of a lower specificity regarding HT and NAITD.
References
Schott M, Scherbaum WA, Morgenthaler NG. Thyrotropin receptor autoantibodies in Graves’ disease. Trends Endocrinol Metab. 2005;16:243–8.
Zöphel K, Roggenbuck D, Schott M. Clinical review about TRAb assay’s history. Autoimmun Rev. 2010;9:695–700.
Diana T, Wuster C, Kanitz M, Kahaly GJ. Highly variable sensitivity of five binding and two bio-assays for TSH-receptor antibodies. J Endocrinol Investig. 2016;39:1159–65.
Morgenthaler NG, Ho SC, Minich WB. Stimulating and blocking thyroid-stimulating hormone (TSH) receptor autoantibodies from patients with Graves’ disease and autoimmune hypothyroidism have very similar concentration, TSH receptor affinity, and binding sites. J Clin Endocrinol Metab. 2007;92:1058–65.
Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev. 1998;19:673–716.
Costagliola S, Morgenthaler NG, Hoermann R, et al. Second generation assay for thyrotropin receptor antibodies has superior diagnostic sensitivity for Graves’ disease. J Clin Endocrinol Metab. 1999;84:90–7.
Shewring G, Smith BR. An improved radioreceptor assay for TSH receptor antibodies. Clin Endocrinol. 1982;17:409–17.
Massart C, Orgiazzi J, Maugendre D. Clinical validity of a new commercial method for detection of TSH-receptor binding antibodies in sera from patients with Graves’ disease treated with antithyroid drugs. Clin Chim Acta. 2001;304:39–47.
Sahlmann CO, Schreivogel I, Angerstein C, et al. Clinical evaluation of a new thyroglobulin immunoradiometric assay in the follow-up of differentiated thyroid carcinoma. Nuklearmedizin. 2003;42:71–7.
Sanders J, Allen F, Jeffreys J, et al. Characteristics of a monoclonal antibody to the thyrotropin receptor that acts as a powerful thyroid-stimulating autoantibody antagonist. Thyroid. 2005;15:672–82.
Villalta D, D’Aurizio F, Da RM, Ricci D, Latrofa F, Tozzoli R. Diagnostic accuracy of a new fluoroenzyme immunoassay for the detection of TSH receptor autoantibodies in Graves’ disease. Auto Immun Highlights. 2018;9:3.
Zöphel K, Roggenbuck D, Wunderlich G, Schott M. Continuously increasing sensitivity over three generations of TSH receptor autoantibody assays. Horm Metab Res. 2010;42:900–2.
Zöphel K, Roggenbuck D, von Landenberg P, et al. TSH receptor antibody (TRAb) assays based on the human monoclonal autoantibody M22 are more sensitive than bovine TSH based assays. Horm Metab Res. 2010;42:65–9.
Shimojo N, Kohno Y, Yamaguchi K, et al. Induction of Graves-like disease in mice by immunization with fibroblasts transfected with the thyrotropin receptor and a class II molecule. Proc Natl Acad Sci U S A. 1996;93:11074–9.
Rees Smith B, Bolton J, Young S, et al. A new assay for thyrotropin receptor autoantibodies. Thyroid. 2004;14:830–5.
Zöphel K, von Landenberg P, Roggenbuck D, Wunderlich G, Kotzerke J, Lackner KJ. Are porcine and human TSH receptor antibody measurements comparable? Clin Lab. 2008:1–8.
Zöphel K, Wunderlich G, Kotzerke J, von Landenberg P, Roggenbuck D. M22 based (manual) ELISA for TSH-receptor antibody (TRAb) measurement is more sensitive than 2nd generation TRAb assays. Clin Chim Acta. 2009;403:266.
Rees SB, Sanders J, Furmaniak J. TSH receptor antibodies. Thyroid. 2007;17:923–38.
Bolton J, Sanders J, Oda Y, et al. Measurement of thyroid-stimulating hormone receptor autoantibodies by ELISA. Clin Chem. 1999;45:2285–7.
Kamijo K. TSH-receptor antibodies determined by the first, second and third generation assays and thyroid-stimulating antibody in pregnant patients with Graves’ disease. Endocr J. 2007;54:619–24.
Sanders J, Evans M, Premawardhana LD, et al. Human monoclonal thyroid stimulating autoantibody. Lancet. 2003;362:126–8.
Frank CU, Braeth S, Dietrich JW, Wanjura D, Loos U. Bridge technology with TSH receptor chimera for sensitive direct detection of TSH receptor antibodies causing Graves’ disease: analytical and clinical evaluation. Horm Metab Res. 2015;47:880–8.
Kahaly GJ, Diana TTSH. Receptor antibody functionality and nomenclature. Front Endocrinol (Lausanne). 2017;8:28.
Allelein S, Ehlers M, Goretzki S, et al. Clinical evaluation of the first automated assay for the detection of stimulating TSH receptor autoantibodies. Horm Metab Res. 2016;48:795–801.
Tozzoli R, Kodermaz G, Villalta D, Bagnasco M, Pesce G, Bizzaro N. Accuracy of receptor-based methods for detection of thyrotropin-receptor autoantibodies: a new automated third-generation immunoassay shows higher analytical and clinical sensitivity for the differential diagnosis of hyperthyroidism. Auto Immun Highlights. 2010;1:95–100.
Araki N, Iida M, Amino N, et al. Rapid bioassay for detection of thyroid-stimulating antibodies using cyclic adenosine monophosphate-gated calcium channel and aequorin. Eur Thyroid J. 2015;4:14–9.
Bergmann A, Struck J. Receptor binding assay for detecting TSH receptor auto-antibodies. B.R.A.H.M.S.AG. PCT/EP1997/006767(EP0943089). 1999. Ref Type: Patent.
Hesarghatta SA, Abraham P. Measuring TSH receptor antibody to influence treatment choices in Graves’ disease. Clin Endocrinol. 2017;86:652–7.
Struja T, Fehlberg H, Kutz A, et al. Can we predict relapse in Graves’ disease? Results from a systematic review and meta-analysis. Eur J Endocrinol. 2017;176:87–97.
Schott M, Morgenthaler NG, Fritzen R, et al. Levels of autoantibodies against human TSH receptor predict relapse of hyperthyroidism in Graves' disease. Horm Metab Res. 2004;36:92–6.
Zöphel K, Gruning T, Roggenbuck D, Wunderlich G, Kotzerke J. On specificity of 2nd generation TSH receptor autoantibody measurements. Clin Lab. 2008;54:243–9.
Schott M, Feldkamp J, Bathan C, Fritzen R, Scherbaum WA, Seissler J. Detecting TSH-receptor antibodies with the recombinant TBII assay: technical and clinical evaluation. Horm Metab Res. 2000;32:429–35.
Kamijo K. TSH-receptor antibody measurement in patients with various thyrotoxicosis and Hashimoto's thyroiditis: a comparison of two two-step assays, coated plate ELISA using porcine TSH-receptor and coated tube radioassay using human recombinant TSH-receptor. Endocr J. 2003;50:113–6.
Kahaly GJ, Diana T, Glang J, Kanitz M, Pitz S, Konig J. Thyroid stimulating antibodies are highly prevalent in Hashimoto’s thyroiditis and associated orbitopathy. J Clin Endocrinol Metab. 2016;101:1998–2004.
Hermsen D, Liu C, Domberg J, et al. Comparison of a solid phase human- versus porcine- thyrotropin receptor-based immunoassay for the measurement of thyrotropin receptor antibodies in patients with thyroid diseases. Exp Clin Endocrinol Diabetes. 2008;116(Suppl 1):S59–63.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DR is a shareholder and has a leading positation in Medipan and GA Generic Assays, both of which are diagnostic manufacturers. All other authors do not declare a conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Roggenbuck, J.J., Veiczi, M., Conrad, K. et al. A novel third-generation TSH receptor antibody (TRAb) enzyme-linked immunosorbent assay based on a murine monoclonal TSH receptor-binding antibody. Immunol Res 66, 768–776 (2018). https://doi.org/10.1007/s12026-018-9062-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-018-9062-z