Abstract
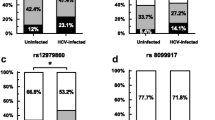
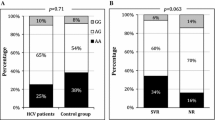
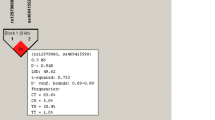
Previous studies showed that interleukin (IL)-28B gene polymorphisms were associated with hepatitis C Virus (HCV) infection and treatment outcomes. We tested whether single-nucleotide polymorphisms (SNPs) in IL-28A and IL-28B are associated with HCV infection among Egyptians with HCV genotype 4 infections. We enrolled 144 chronic HCV patients, 72 spontaneously resolved HCV subjects, and 69 healthy controls. Four SNPs in IL-28A and IL-28B genes (IL-28A.rs12980602, IL-28B.rs12979860, IL-28B.rs8099917, and IL-28B.rs8103142) were genotyped. The most frequent IL-28B haplotype “TCT” was significantly more frequent in HCV-infected subjects than in HCV negative subjects (62.2% vs. 48.6%, respectively; p = 0.005). The frequency of IL-28A.rs12980602 “T” allele was significantly higher than the “C” allele in healthy controls compared to HCV-infected subjects (p < 0.001) with the “TT” genotype significantly higher in healthy controls compared to HCV-infected subjects (p < 0.001) with no association with viral load (p = 0.11) among chronically infected subjects. The results, also, confirmed the previous role of IL-28B SNPs in predicting HCV infection outcome. Importantly, IL-28B.rs8099917 “TT” genotype was significantly associated with low viral load in HCV-infected subjects, while the remaining three SNPs did not. The three IL-28B SNPs were in linkage disequilibrium (D′ > 0.68; r2 > 0.43) for all comparisons in HCV patients, while there was no linkage disequilibrium of IL-28A polymorphisms and the three IL-28B SNPs. In conclusion, IL-28A.rs12980602 and IL-28B.rs8103142 TT genotype could be protective against HCV infection. Also, IL-28B.rs12979860, IL-28B.rs8099917, and IL-28B.rs8103142 SNPs predicted the outcome of HCV infection among genotype-4-infected Egyptians. Moreover, IL-28B.rs8099917 SNP affected the viral load in chronic HCV patients.

Similar content being viewed by others
References
Collaborators TPOH. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–76.
WHO. Hepatitis C [fact sheet]. Geneva: World Health Organization; 2016.
Kandeel A, Genedy M, El-Refai S, Funk AL, Fontanet A, Talaat M. The prevalence of hepatitis C virus infection in Egypt 2015: implications for future policy on prevention and treatment. Liv Int. 2017;37(1):45–53. https://doi.org/10.1111/liv.13186.
Ministry of Health E, El-Zanaty and Associates, Egypt and ICF International. Egypt Health Issues Survey. Cairo, Egypt and Rockville: Ministry of Health and ICF International; 2015. p. 2015.
Simmonds P, Holmes EC, Cha TA, Chan SW, McOmish F, Irvine B, et al. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993;74(Pt 11):2391–9.
El-Zayadi A, Selim O, Dabbous HK, Bianchi F. High frequency of smooth muscle antibodies (SMA) among HCV positive chronic liver disease patients in Egypt. J Egypt Public Health Assoc. 1994;69(3–4):205–11.
Abdel-Hamid M, El-Daly M, Molnegren V, El-Kafrawy S, Abdel-Latif S, Esmat G, et al. Genetic diversity in hepatitis C virus in Egypt and possible association with hepatocellular carcinoma. J Gen Virol. 2007;88(Pt 5):1526–31. https://doi.org/10.1099/vir.0.82626-0.
Abdelwahab S, Rewisha E, Hashem M, Sobhy M, Galal I, Allam WR, et al. Risk factors for hepatitis C virus infection among Egyptian healthcare workers in a national liver diseases referral centre. Trans R Soc Trop Med Hyg. 2012;106(2):98–103. https://doi.org/10.1016/j.trstmh.2011.10.003.
El-Zanaty FWA. Egypt demographic and health survey 2008. Cairo: Ministry of Health, El-Zanaty and Associates and Macro International; 2009. p. 431.
Guerra J, Garenne M, Mohamed MK, Fontanet A. HCV burden of infection in Egypt: results from a nationwide survey. J Viral Hepat. 2012;19(8):560–7. https://doi.org/10.1111/j.1365-2893.2011.01576.x.
MHP, El-Zanaty F, ICF. Ministry of Health and Population [Egypt], El-Zanaty and Associates [Egypt], ICF International, Egypt health issues survey 2015. Cairo, Rockville: Ministry of Health and Population, ICF International; 2015.
Elgharably A, Gomaa AI, Crossey MME, Norsworthy PJ, Waked I, Taylor-Robinson SD. Hepatitis C in Egypt – past, present, and future. Int J Gen Med. 2017;10:1–6. https://doi.org/10.2147/IJGM.S119301.
El-Akel W, El-Sayed MH, El Kassas M, El-Serafy M, Khairy M, Elsaeed K, et al. National treatment programme of hepatitis C in Egypt: hepatitis C virus model of care. J Viral Hepat. 2017;24(4):262–7. https://doi.org/10.1111/jvh.12668.
Ayoub HH, Abu-Raddad LJ. Impact of treatment on hepatitis C virus transmission and incidence in Egypt: a case for treatment as prevention. J Viral Hepat. 2017;24(6):486–95. https://doi.org/10.1111/jvh.12671.
Kouyoumjian SP, Chemaitelly H, Abu-Raddad LJ. Characterizing hepatitis C virus epidemiology in Egypt: systematic reviews, meta-analyses, and meta-regressions. Sci Rep. 2018;8(1):1661. https://doi.org/10.1038/s41598-017-17936-4.
Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284(4):450–6.
Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5(3):215–29. https://doi.org/10.1038/nri1573.
Balagopal A, Thomas DL, Thio CL. IL28B and the control of hepatitis C virus infection. Gastroenterology. 2010;139(6):1865–76.
McFarland AP, Horner SM, Jarret A, Joslyn RC, Bindewald E, Shapiro BA, et al. The favorable IFNL3 genotype escapes mRNA decay mediated by AU-rich elements and hepatitis C virus-induced microRNAs. Nat Immunol. 2014;15(1):72–9.
Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4(1):63–8. https://doi.org/10.1038/ni873.
Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4(1):69–77. https://doi.org/10.1038/ni875.
Donnelly RP, Sheikh F, Kotenko SV, Dickensheets H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J Leukoc Biol. 2004;76(2):314–21. https://doi.org/10.1189/jlb.0204117.
Brand S, Dambacher J, Beigel F, Zitzmann K, Heeg MH, Weiss TS, et al. IL-22-mediated liver cell regeneration is abrogated by SOCS-1/3 overexpression in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;292(4):G1019–28. https://doi.org/10.1152/ajpgi.00239.2006.
Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27(4):647–59. https://doi.org/10.1016/j.immuni.2007.07.023.
Hou W, Wang X, Ye L, Zhou L, Yang ZQ, Riedel E, et al. Lambda interferon inhibits human immunodeficiency virus type 1 infection of macrophages. J Virol. 2009;83(8):3834–42. https://doi.org/10.1128/jvi.01773-08.
Almeida GM, de Oliveira DB, Magalhaes CL, Bonjardim CA, Ferreira PC, Kroon EG. Antiviral activity of type I interferons and interleukins 29 and 28a (type III interferons) against Apeu virus. Antivir Res. 2008;80(3):302–8. https://doi.org/10.1016/j.antiviral.2008.06.016.
Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, et al. IL-28A and IL-29 mediate antiproliferative and antiviral signals in intestinal epithelial cells and murine CMV infection increases colonic IL-28A expression. Am J Physiol Gastrointest Liver Physiol. 2005;289(5):G960–8. https://doi.org/10.1152/ajpgi.00126.2005.
Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80(9):4501–9. https://doi.org/10.1128/jvi.80.9.4501-4509.2006.
Robek MD, Boyd BS, Chisari FV. Lambda interferon inhibits hepatitis B and C virus replication. J Virol. 2005;79(6):3851–4. https://doi.org/10.1128/jvi.79.6.3851-3854.2005.
Zhu H, Butera M, Nelson DR, Liu C. Novel type I interferon IL-28A suppresses hepatitis C viral RNA replication. Virol J. 2005;2:80. https://doi.org/10.1186/1743-422x-2-80.
Diegelmann J, Beigel F, Zitzmann K, Kaul A, Goke B, Auernhammer CJ, et al. Comparative analysis of the lambda-interferons IL-28A and IL-29 regarding their transcriptome and their antiviral properties against hepatitis C virus. PLoS One. 2010;5(12):e15200. https://doi.org/10.1371/journal.pone.0015200.
Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401.
Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O'Huigin C, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461(7265):798–801. https://doi.org/10.1038/nature08463.
Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41(10):1105–9.
Kurbanov F, Abdel-Hamid M, Latanich R, Astemborski J, Mohamed M, Mikhail NM, et al. Genetic polymorphism in IL28B is associated with spontaneous clearance of hepatitis C virus genotype 4 infection in an Egyptian cohort. J Infect Dis. 2011;204(9):1391–4.
Pasha HF, Radwan MI, Hagrass HA, Tantawy EA, Emara MH. Cytokines genes polymorphisms in chronic hepatitis C: impact on susceptibility to infection and response to therapy. Cytokine. 2013;61(2):478–84.
Derbala M, Rizk NM, Al-Kaabi S, John A, Sharma M, El-dweik N, et al. The predictive value of IL28B rs12979860, rs11881222 and rs8099917 polymorphisms and IP-10 in the therapeutic response of Egyptian genotype 4 patients. Virology. 2013;444(1–2):292–300.
Pedergnana V, Abdel-Hamid M, Guergnon J, Mohsen A, Le Fouler L, Theodorou I, et al. Analysis of IL28B variants in an Egyptian population defines the 20 kilobases minimal region involved in spontaneous clearance of hepatitis C virus. PLoS One. 2012;7(6):e38578.
Asselah T. A revolution in HCV treatment with direct-acting antivirals: from non-response to eradication. J Hepatol. 2012;57(2):455–7.
Asselah T, De Muynck S, Broet P, Masliah-Planchon J, Blanluet M, Bieche I, et al. IL28B polymorphism is associated with treatment response in patients with genotype 4 chronic hepatitis C. J Hepatol. 2012;56(3):527–32.
Knapp S, Warshow U, Ho KMA, Hegazy D, Little AM, Fowell A, et al. A polymorphism in IL28B distinguishes exposed, uninfected individuals from spontaneous resolvers of HCV Infection. Gastroenterology. 2011;141(1):320–5.e2. https://doi.org/10.1053/j.gastro.2011.04.005.
Knapp S, Meghjee N, Cassidy S, Jamil K, Thursz M. Detection of allele specific differences in IFNL3 (IL28B) mRNA expression. BMC Med Genet. 2014;15:104. https://doi.org/10.1186/s12881-014-0104-7.
Cheng H-R, Liu C-J, Tseng T-C, Su T-H, Yang H-I, Chen C-J, et al. Host genetic factors affecting spontaneous HBsAg seroclearance in chronic hepatitis B patients. PLoS One. 2013;8(1):e53008. https://doi.org/10.1371/journal.pone.0053008.
Lane J, McLaren PJ, Dorrell L, Shianna KV, Stemke A, Pelak K, et al. A genome-wide association study of resistance to HIV infection in highly exposed uninfected individuals with hemophilia a. Hum Mol Genet. 2013;22(9):1903–10. https://doi.org/10.1093/hmg/ddt033.
Morgan TR, Lambrecht RW, Bonkovsky HL, Chung RT, Naishadham D, Sterling RK, et al. DNA polymorphisms and response to treatment in patients with chronic hepatitis C: results from the HALT-C trial. J Hepatol. 2008;49(4):548–56. https://doi.org/10.1016/j.jhep.2008.05.011.
Mangia A, Santoro R, Copetti M, Massari M, Piazzolla V, Spada E, et al. Treatment optimization and prediction of HCV clearance in patients with acute HCV infection. J Hepatol. 2013;59(2):221–8. https://doi.org/10.1016/j.jhep.2013.04.007.
Zhang L, Jilg N, Shao RX, Lin W, Fusco DN, Zhao H, et al. IL28B inhibits hepatitis C virus replication through the JAK-STAT pathway. J Hepatol. 2011;55(2):289–98. https://doi.org/10.1016/j.jhep.2010.11.019.
Dolganiuc A, Kodys K, Marshall C, Saha B, Zhang S, Bala S, et al. Type III interferons, IL-28 and IL-29, are increased in chronic HCV infection and induce myeloid dendritic cell-mediated FoxP3+ regulatory T cells. PLoS One. 2012;7(10):e44915. https://doi.org/10.1371/journal.pone.0044915.
Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–4.
Urban TJ, Thompson AJ, Bradrick SS, Fellay J, Schuppan D, Cronin KD, et al. IL28B genotype is associated with differential expression of intrahepatic interferon-stimulated genes in patients with chronic hepatitis C. Hepatology (Baltimore, MD). 2010;52(6):1888–96. https://doi.org/10.1002/hep.23912.
Smith KR, Suppiah V, O'Connor K, Berg T, Weltman M, Abate ML, et al. Identification of improved IL28B SNPs and haplotypes for prediction of drug response in treatment of hepatitis C using massively parallel sequencing in a cross-sectional European cohort. Genome Med. 2011;3(8):57. https://doi.org/10.1186/gm273.
Jin G, Kang H, Chen X, Dai D. Evaluation of the relationship between IL28B, IL10RB and IL28RA single-nucleotide polymorphisms and susceptibility to hepatitis C virus in Chinese Han population. Infect Genet Evol. 2014;21:8–14. https://doi.org/10.1016/j.meegid.2013.10.009.
Di Marco V, Bronte F, Calvaruso V, Capra M, Borsellino Z, Maggio A, et al. IL28B polymorphisms influence stage of fibrosis and spontaneous or interferon-induced viral clearance in thalassemia patients with hepatitis C virus infection. Haematologica. 2012;97(5):679–86. https://doi.org/10.3324/haematol.2011.050351.
Zhang AM, Ma K, Song Y, Wang B, Feng Y, Liu L, et al. Genetic polymorphisms of the IFNlambda genes are associated with biochemical features in Han Chinese with HCV infection from Yunnan Province, China. Infect Genet Evol. 2014;21:161–5. https://doi.org/10.1016/j.meegid.2013.11.013.
Yu F, Wang Y, Yuan S, Ma J, Ma N, Zhang X, et al. Association between gene polymorphisms of IL-28 and response to lamivudine in Chinese rural patients with chronic hepatitis B. Scandinavian Journal of Gast Roenterology. 2013;48:745–51.
Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138(1338–1345):1345.e1331–7.
Tipu I, Marriage F, Farooqi Z-U-R, Platt H, Athar MA, Day PJ, et al. The IFN-λ genetic polymorphism association with the viral clearance induced by hepatitis c virus treatment in Pakistani patients. Hepatitis Monthly. 2014;14(3):e15076. https://doi.org/10.5812/hepatmon.15076.
About F, Oudot-Mellakh T, Niay J, Rabiéga P, Pedergnana V, Duffy D. Impact of IL28B, APOH and ITPA polymorphisms on efficacy and safety of TVRor BOC-based triple therapy in treatment-experienced HCV-1 patients with compensated cirrhosis from the ANRS CO20-CUPIC study. PLoS One. 2015;10. https://doi.org/10.1371/journal.pone.0145105.
Barreiro P, Vispo E, Poveda E, Fernández-Montero JV, Soriano V. Hepatitis C therapy: highlights from the 2012 annual meeting of the European Association for the Study of the liver. Clin Infect Dis. 2013;56. https://doi.org/10.1093/cid/cis915.
Calisti G, Tavares A, Macartney MJ, McCormick A, Labbett W, Jacobs M. IL28B genotype predicts response to chronic hepatitis C triple therapy with telaprevir or boceprevir in treatment naïve and treatment-experienced patients other than prior partial- and null-responders. Spring 2015;4. doi:https://doi.org/10.1186/s40064-015-1137-x.
D’Offizi G, Cammà C, Taibi C, Schlag M, Palma M, Demasi R. Clinical and virological predictors of sustained response with an interferon-based simeprevir regimen for patients with chronic genotype 1 hepatitis C virus infection. New Microbiol 2017;40(1):19–26.
Holmes JA, Desmond PV, Thompson AJ. Does IL28B genotyping still have a role in the era of direct-acting antiviral therapy for chronic hepatitis C infection? J Viral Hepat 2012;19(10):677–84.
Akuta N, Sezaki H, Suzuki F, Fujiyama S, Kawamura Y, Hosaka T. Retreatment efficacy and predictors of ledipasvir plus sofosbuvir to HCV genotype 1 in Japan. J Med Virol 2017;89(2):284–90.
Echeverría N, Chiodi D, López P, Sanchez Ciceron A, Angulo J, López-Lastra M, et al. IL28B gene polymorphism rs12979860, but not rs8099917, contributes to the occurrence of chronic HCV infection in Uruguayan patients. Virol J. 2018;15(1):40. https://doi.org/10.1186/s12985-018-0946-2.
Abdelwahab SF, Zakaria Z, Allam WR, Hamdy S, Mahmoud MA, Sobhy M, et al. Interleukin 28B.rs12979860 genotype does not affect hepatitis C viral load in Egyptians with genotype 4 chronic infection. Arch Virol. 2015;160(11):2833–7. https://doi.org/10.1007/s00705-015-2555-3.
Halfon P, Bourliere M, Ouzan D, Maor Y, Renou C, Wartelle C, et al. A single IL28B genotype SNP rs12979860 determination predicts treatment response in patients with chronic hepatitis C genotype 1 virus. Eur J Gastroenterol Hepatol. 2011;23(10):931–5. https://doi.org/10.1097/MEG.0b013e328349d0ef.
Bochud PY, Bibert S, Negro F, Haagmans B, Soulier A, Ferrari C, et al. IL28B polymorphisms predict reduction of HCV RNA from the first day of therapy in chronic hepatitis C. J Hepatol. 2011;55(5):980–8. https://doi.org/10.1016/j.jhep.2011.01.050.
Acknowledgments
We would like to thank Dr. Nabiel Mikhail and Enas S. Aziz (Egyblood) for their technical help with the data. We thank Mrs. Hoayda M. Ahmed and Dr. Mohamed Abdel-Samee (from the NLI) for their assistance with sample collection and enrollment of the subjects. We particularly appreciate Dr. Gehan Galal (Director of Egyblood R&D Department), Dr. Nelly Sedky, and Dr. Hala Hussein (previous Egyblood CEOs,) for their support throughout the conduct of the study.
Funding
This study was supported by the European Union 6th Framework Program contract no. 0374435 to the HEPACIVAC consortium and by the European Union 7th Framework Program contract no. 260844 to the HEPACUTE consortium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was approved by the NLI Institutional Review Board.
Informed consent
Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Zakaria, Z.A., Knapp, S., Hashem, M. et al. Interleukin 28A.rs12980602 and interleukin 28B.rs8103142 genotypes could be protective against HCV infection among Egyptians. Immunol Res 67, 123–133 (2019). https://doi.org/10.1007/s12026-018-9035-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-018-9035-2