Abstract
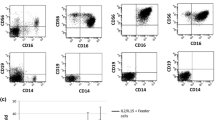
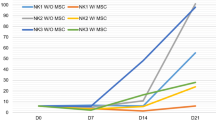
The ability of natural killer (NK) cells to kill tumor cells without antigen recognition makes them appealing as an adoptive immunotherapy. However, NK cells are not routinely used in the context of leukemic relapse after hematopoietic stem cell transplantation. Patients who experience relapse can be treated with donor lymphocyte infusions (DLI) based on small-cell fractions frozen at the time of transplantation. Since peripheral blood stem cells (PBSCs) are increasingly used as a stem cell source and as a source of cells for DLI, we aimed to evaluate the impact of G-SCF mobilization on NK cell phenotype, subset repartition, and functionality. Immunomagnetically isolated NK cells from healthy donor blood, donor PBSCs, and patient PBSCs were expanded for 14 days with IL-15. The expansion capacity, phenotype, and functions (cytokine secretion and cytotoxicity) of NK cell subsets based on CD56 and CD16 expression were then evaluated. Mobilized sources showed a significant decrease of CD56brightCD16+ NK cells (28 versus 74%), whereas a significant increase (64 versus 15%) of CD56brightCD16− NK cells was observed in comparison with peripheral blood. Patient-mobilized NK cells showed a significantly decreased cytotoxicity, and antibody-dependent cell cytototoxicity (ADCC) was also observed to a lesser extent in NK cells from healthy donor PBSC. G-CSF-mobilized NK cell TNF-α and IFN-γ secretion was impaired at day 0 compared to healthy donors but was progressively restored after culture. In conclusion, expansion of NK cells from G-CSF-mobilized sources may progressively improve their functionality.




Similar content being viewed by others
References
Liu YC, Chien SH, Fan NW, Hu MH, Gau JP, Liu CJ, et al. Prognostic factors on the graft-versus-host disease-free and relapse-free survival after adult allogeneic hematopoietic stem cell transplantation. Stem Cells Int. 2016;2016:5143071.
Bar M, et al. Donor lymphocyte infusion for relapsed hematological malignancies after allogeneic hematopoietic cell transplantation: prognostic relevance of the initial CD3+ T cell dose. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2013;19(6):949–57.
Kolb HJ, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76(12):2462–5.
Costa-Garcia M, Vera A, Moraru M, Vilches C, López-Botet M, Muntasell A, et al. Antibody-mediated response of NKG2Cbright NK cells against human cytomegalovirus. J Immunol Baltim Md 1950. 2015;194(6):2715–24.
Haen SP, et al. Prognostic relevance of HER2/neu in acute lymphoblastic leukemia and induction of NK cell reactivity against primary ALL blasts by trastuzumab. Oncotarget. 2016;7(11):13013–30.
Ruggeri L, et al. NK cell alloreactivity and allogeneic hematopoietic stem cell transplantation. Blood Cells Mol Dis. 2008;40(1):84–90.
Lanier LL, Chang C, Azuma M, Ruitenberg JJ, Hemperly JJ, Phillips JH. Molecular and functional analysis of human natural killer cell-associated neural cell adhesion molecule (N-CAM/CD56). J. Immunol. Baltim. Md 1950. 1991;146(12):4421–6.
Michel T, et al. Human CD56bright NK cells: an update. J. Immunol. Baltim. Md 1950. 2016;196(7):2923–31.
Béziat V, et al. CD56brightCD16+ NK cells: a functional intermediate stage of NK cell differentiation. J. Immunol. Baltim. Md 1950. 2011;186(12):6753–61.
Velardi A. Role of KIRs and KIR ligands in hematopoietic transplantation. Curr Opin Immunol. 2008;20(5):581–7.
Hsu KC, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105(12):4878–84.
Schmid C, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(31):4938–45.
Rondelli D, et al. Alloantigen presenting capacity, T cell alloreactivity and NK function of G-CSF-mobilized peripheral blood cells. Bone Marrow Transplant. 1998;22(7):631–7.
Su Y-C, et al. G-CSF downregulates natural killer cell-mediated cytotoxicity in donors for hematopoietic SCT. Bone Marrow Transplant. 2012;47(1):73–81.
Decot V, et al. Natural-killer cell amplification for adoptive leukemia relapse immunotherapy: comparison of three cytokines, IL-2, IL-15, or IL-7 and impact on NKG2D, KIR2DL1, and KIR2DL2 expression. Exp Hematol. 2010;38(5):351–62.
Bogunia-Kubik K, Gieryng A, Gebura K, Lange A, et al. Genetic variant of the G-CSF receptor gene is associated with lower mobilization potential and slower recovery of granulocytes after transplantation of autologous peripheral blood progenitor cells. Cytokine. 2012;60(2):463–7.
Saito M, et al. Granulocyte colony-stimulating factor directly affects human monocytes and modulates cytokine secretion. Exp Hematol. 2002;30(10):1115–23.
Toh HC, et al. G-CSF induces a potentially tolerant gene and immunophenotype profile in T cells in vivo. Clin Immunol Orlando Fla. 2009;132(1):83–92.
Miller JS, Prosper F, McCullar V. Natural killer (NK) cells are functionally abnormal and NK cell progenitors are diminished in granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cell collections. Blood. 1997;90(8):3098–105.
Trzonkowski P, et al. Differences in kinetics of donor lymphoid cells in response to G-CSF administration may affect the incidence and severity of acute GvHD in respective HLA-identical sibling recipients. Med Oncol Northwood Lond Engl. 2004;21(1):81–94.
Tayebi H, et al. Effect of granulocyte colony-stimulating factor mobilization on phenotypical and functional properties of immune cells. Exp Hematol. 2001;29(4):458–70.
Taga T, Kariya Y, Shimada M, Uchida A. Suppression of natural killer cell activity by granulocytes in patients with aplastic anemia: role of granulocyte colony-stimulating factor. Immunol Lett. 1993;39(1):65–70.
Baessler T, et al. CD137 ligand mediates opposite effects in human and mouse NK cells and impairs NK-cell reactivity against human acute myeloid leukemia cells. Blood. 2010;115(15):3058–69.
Lv M, et al. Monocytic and promyelocytic myeloid-derived suppressor cells may contribute to G-CSF-induced immune tolerance in haplo-identical allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2015;90(1):E9–E16.
Vaknin I, et al. A common pathway mediated through Toll-like receptors leads to T- and natural killer-cell immunosuppression. Blood. 2008;111(3):1437–47.
Siegler U, et al. Activated natural killer cells from patients with acute myeloid leukemia are cytotoxic against autologous leukemic blasts in NOD/SCID mice. Leukemia. 2005;19(12):2215–22.
Funding
This study was supported by an Overseas Study Fellowship from the China Council, [http://www.csc.edu.cn/], no. 201308420488 and the Direction de la Recherche Clinique of the CHRU of Nancy.
Author information
Authors and Affiliations
Contributions
YX performed the experiments. MM performed the ADCC assay. LR and DB read and correct the paper. JFS provides advices on the paper. CQ designed the ADCC assay. VD designs the experiments and writes and submits the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Xiong, Y., Mouginot, M., Reppel, L. et al. Modification of NK cell subset repartition and functions in granulocyte colony-stimulating factor-mobilized leukapheresis after expansion with IL-15. Immunol Res 65, 1130–1138 (2017). https://doi.org/10.1007/s12026-017-8955-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-017-8955-6