Abstract
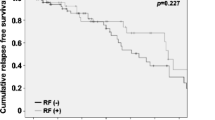
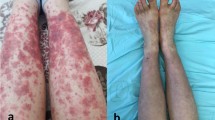
This is the largest direct immunofluorescence (DIF) analysis of patients with histology-proven cutaneous leukocytoclastic vasculitis (LCV). To establish the correlation of deposition of immune complexes at the blood vessel walls with underlying causes and prognosis of LCV, we performed a retrospective study from January 2007 to December 2014. The patients are followed at the Department of Dermatology, Hospital Das Clínicas da Faculdade de Medicina da Universidade de São Paulo, a tertiary hospital at São Paulo, Brazil. We reviewed the data of 282 biopsy-proven LCV cases with DIF performed. For the statistical analysis, we included only patients with positive DIF exclusively in vessel walls (235/282 patients). We planned to find a correlation between the DIF profiles of LCV patients and the epidemiology data, underlying causes and prognosis. Ages ranged from five to 87 years old (yo), median age of 45 and 191/282 (67.73 %) were female individuals. DIF analysis showed positivity in 70.21 % of the samples, and C3 was the most frequent immunoreactant. Immunoglobulin A (IgA) deposition at the blood vessel wall was related to age and absence of autoimmune/inflammatory diseases. Immunoglobulin M (IgM) deposition at the blood vessel wall was related to females, autoimmune/inflammatory disorders, C3 and C4 consumption and antinuclear antibody and anti-SSA/anti-SSB positivity. Immunoglobulin G (IgG) deposition at the blood vessel wall was associated with age and positive ANCA; finally, C3 deposition at the blood vessel wall was associated with hematuria and renal involvement. Systemic involvement was present in 12.5 % cases of LCV patients. C3 deposits, the most frequent finding of this study, were related to renal involvement; IgA deposits to absence of autoimmune or inflammatory diseases; IgM deposition to the presence of autoimmune or inflammatory diseases and IgG deposits were associated with positive ANCA. DIF seems to be an important method to establish the prognosis and underlying etiology of LCV. Characterization of the immune complex at the blood vessel wall by DIF is relevant to determine underlying conditions related to LCV.

Similar content being viewed by others
References
Lotti T, Ghersetich I, Comacchi C, Jorizzo JL. Cutaneous small-vessel vasculitis. J Am Acad Dermatol. 1998;39(5 Pt 1):667–87.
Tosca N, Stratigos JD. Possible pathogenetic mechanisms in allergic cutaneous vasculitis. Int J Dermatol. 1988;27(5):291–6.
Minz RW, Chhabra S, Singh S, Radotra BD, Kumar B. Direct immunofluorescence of skin biopsy: perspective of an immunopathologist. Indian J Dermatol Venereol Leprol. 2010;76(2):150–7.
Takeuchi S, Soma Y, Kawakami T. IgM in lesional skin of adults with Henoch-Schönlein purpura is an indication of renal involvement. J Am Acad Dermatol. 2010;63(6):1026–9.
Barnadas MA, Pérez E, Gich I, et al. Diagnostic, prognostic and pathogenic value of the direct immunofluorescence test in cutaneous leukocytoclastic vasculitis. Int J Dermatol. 2004;43(1):19–26.
Alalwani M, Billings SD, Gota CE. Clinical significance of immunoglobulin deposition in leukocytoclastic vasculitis: a 5-year retrospective study of 88 patients at cleveland clinic. Am J Dermatopathol. 2014;36(9):723–9.
Zurada JM, Ward KM, Grossman ME. Henoch-Schönlein purpura associated with malignancy in adults. J Am Acad Dermatol. 2006;55(5 Suppl):S65–70.
Gibson LE. Cutaneous vasculitis update. Dermatol Clin. 2001;19(4):603–15.
Russell JP, Gibson LE. Primary cutaneous small vessel vasculitis: approach to diagnosis and treatment. Int J Dermatol. 2006;45(1):3–13.
Sais G, Vidaller A, Jucglà A, Servitje O, Condom E, Peyri J. Prognostic factors in leukocytoclastic vasculitis: a clinicopathologic study of 160 patients. Arch Dermatol. 1998;134(3):309–15.
Linskey KR, Kroshinsky D, Mihm MC, Hoang MP. Immunoglobulin-A–associated small-vessel vasculitis: a 10-year experience at the Massachusetts General Hospital. J Am Acad Dermatol. 2012;66(5):813–22.
Belli AA, Dervis E. The correlation between cutaneous IgM deposition and renal involvement in adult patients with Henoch–Schönlein purpura. Eur J Dermatol. 2014;24(1):81–4.
Mackel SE, Jordon RE. Leukocytoclastic vasculitis. A cutaneous expression of immune complex disease. Arch Dermatol. 1982;118(5):296–301.
Sanchez NP, Van Hale HM, Su WP. Clinical and histopathologic spectrum of necrotizing vasculitis. Report of findings in 101 cases. Arch Dermatol. 1985;121(2):220–4.
Merrill J, Lahita RG. Henoch-Schönlein purpura remitting in pregnancy and during sex steroid therapy. Br J Rheumatol. 1994;33(6):586–8.
Joseph G, Holtman JS, Kosfeld RE, Blodgett WA, Liu YK. Pregnancy in Henoch-Schönlein purpura. Am J Obstet Gynecol. 1987;157(4 Pt 1):911–2.
Goldstein AR, White RH, Akuse R, Chantler C. Long-term follow-up of childhood Henoch-Schönlein nephritis. Lancet. 1992;339(8788):280–2.
Koizumi M, Hagino D, Fukuyama C, et al. Schönlein-Henoch purpura during pregnancy: case report and review of the literature. J Obstet Gynaecol Res. 2004;30(1):37–41.
Borahay MA, Kelly BC, Harirah HM. Systemic lupus erythematosus presenting with leukocytoclastic vasculitis and seizure during pregnancy. Am J Perinatol. 2009;26(6):431–5.
Tomizawa K, Sato-Matsumura KC, Kajii N. The coexistence of cutaneous vasculitis and thrombosis in childhood-onset systemic lupus erythematosus with antiphospholipid antibodies. Br J Dermatol. 2003;149(2):439–41.
Sánchez-Pérez J, Peñas PF, Ríos-Buceta L, Fernández-Herrera J, Fraga J, García-Díez A. Leukocytoclastic vasculitis in subacute cutaneous lupus erythematosus: clinicopathologic study of three cases and review of the literature. Dermatology. 1996;193(3):230–5.
Yasue T. Livedoid vasculitis and central nervous system involvement in systemic lupus erythematosus. Arch Dermatol. 1986;122(1):66–70.
Oien RF, Håkansson A, Hansen BU. Leg ulcers in patients with rheumatoid arthritis—a prospective study of aetiology, wound healing and pain reduction after pinch grafting. Rheumatology (Oxford). 2001;40(7):816–20.
Westedt ML, Meijer CJ, Vermeer BJ, Cats A, de Vries E. Rheumatoid arthritis—the clinical significance of histo- and immunopathological abnormalities in normal skin. J Rheumatol. 1984;11(4):448–53.
Carlson JA, Chen KR. Cutaneous vasculitis update: neutrophilic muscular vessel and eosinophilic, granulomatous, and lymphocytic vasculitis syndromes. Am J Dermatopathol. 2007;29(1):32–43.
Brons RH, de Jong MC, de Boer NK, Stegeman CA, Kallenberg CG, Tervaert JW. Detection of immune deposits in skin lesions of patients with Wegener’’ granulomatosis. Ann Rheum Dis. 2001;60(12):1097–102.
Sánchez-Guerrero J, Gutiérrez-Ureña S, Vidaller A, Reyes E, Iglesias A, Alarcón-Segovia D. Vasculitis as a paraneoplastic syndrome. Report of 11 cases and review of the literature. J Rheumatol. 1990;17(11):1458–62.
García-Porrúa C, González-Gay MA. Cutaneous vasculitis as a paraneoplastic syndrome in adults. Arthritis Rheum. 1998;41(6):1133–5.
Fain O, Hamidou M, Cacoub P, et al. Vasculitides associated with malignancies: analysis of sixty patients. Arthritis Rheum. 2007;57(8):1473–80.
Pertuiset E, Lioté F, Launay-Russ E, Kemiche F, Cerf-Payrastre I, Chesneau AM. Adult Henoch-Schönlein purpura associated with malignancy. Semin Arthritis Rheum. 2000;29(6):360–7.
Podjasek JO, Wetter DA, Pittelkow MR, Wada DA. Henoch-Schönlein purpura associated with solid-organ malignancies: three case reports and a literature review. Acta Derm Venereol. 2012;92(4):388–92.
Acknowledgments
We are indebted to Daniel Silvestre—LIM01 University of São Paulo Medical School, Brazil—for the help with the statistical analysis.
Author Contributions
Dr(s) Heringer and Takatu had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Heringer, Takatu, Criado, Aoki, Valente, and Sanchez contributed to study concept and design. Heringer, Takatu, and Sanchez contributed to acquisition, analysis, and interpretation of data, and drafting of the manuscript. Aoki, Criado, Valente, and Carvalho critically revised the manuscript for important intellectual content. Daniel Silvestre performed the statistical analysis. No funding was obtained. Aoki and Criado provided administrative, technical, or material support. Aoki, Valente, and Criado supervised the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Takatu, C.M., Heringer, A.P.R., Aoki, V. et al. Clinicopathologic correlation of 282 leukocytoclastic vasculitis cases in a tertiary hospital: a focus on direct immunofluorescence findings at the blood vessel wall. Immunol Res 65, 395–401 (2017). https://doi.org/10.1007/s12026-016-8850-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-016-8850-6