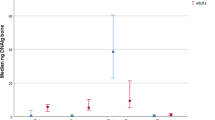
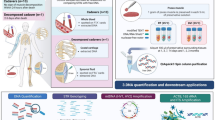
Abstract
The use of DNA extracted from skeletonized human remains is a common challenge for those working in human identification. Thermal age and chemical compromise should be considered prior to performing DNA testing on skeletonized remains. Both heat and chemical contamination may cause damage to the DNA present in the osseous materials and a subsequent increase in both the difficulty and expense of DNA testing. For this study, three World War II era mass fatality events involving the US military, the USS Oklahoma, the Battle of Tarawa, and the Cabanatuan Prison Camps, were examined for the overall success of DNA testing using five DNA modalities: Sanger sequencing of mitochondrial DNA, AmpFlSTR® MiniFiler™; PowerPlex® Fusion; a modified AmpFlSTR® Yfiler™; and a Next Generation Sequencing (NGS) protocol. Decedents from the three chosen incidents were buried in tropical environments and have the same approximate post mortem interval of 75 years, however, the chemical conditions that decedents were exposed to at each of the incidents vary. Remains from the USS Oklahoma were soaked in fuel oil and salt water immediately post-mortem; Cabanatuan Prison Camp remains were treated with a ‘hardening’ compound; and those from the Battle of Tarawa were not treated. Skeletal elements from each incident were compared across the 5 tested DNA modalities for success. Chemical insult to skeletal materials appears to have the greatest impact on every modality of DNA testing examined.



Similar content being viewed by others
References
Galloway A, Willy P, Snyder L. Human bone mineral densities and survival of bone elements: a contemporary sample. In: Haglund WD, Sorg MH, editors. Forensic taphonomy: the postmortem fate of human remains. Boca Raton, FL: CRC Press; 1997. p. 295–317.
Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–15.
Smith CI, Chamerlain AT, Riley MS, Stringer C, Collins MJ. The thermal history of fossils and the likelihood of successful DNA amplification. J Hum Evol. 2003;45:203–17.
Hofreiter M, Paijmans JLA, Goodchild H, Speller CF, Barlow A, Fortes GG, et al. The future of ancient DNA: Technical advances and conceptual shifts. Bioessays. 2014;37:284–93.
Nieves-Colón MA, Ozga AT, Pestle WJ, Cucina A, Tiesler V, Stanton TW, et al. Comparison of two ancient DNA extraction protocols for skeletal remains from tropical environments. Am J Phys Anth. 2018;166:824–36.
Holland MM, Cave CA, Holland CA, Bille TW. Development of a quality, high throughput DNA analysis procedure for skeletal samples to assist with the identification of victims from the World Trade Center attacks. Croat Med J. 2003;44:264–72.
Mundorff AZ, Bartelink EJ, Mar-Cash E. DNA preservation in skeletal elements from the World Trade Center disaster: Recommendations for mass fatality management. J For Sci. 2009;54:739–45.
Sutlović D, Gojanivić MD, Anđelnović S. Rapid extraction of human DNA containing humic acid. Croat Chem Acta. 2007;80:117–20.
Nicholson GJ, Tomiuk J, Czarnetzki A, Bachmann L, Pusch CM. Detection of bone glue treatment as a major source of contamination in ancient DNA analyses. Am J Phys Anth. 2002;118:117–20.
National Archives. Identifier: 6919514. Local Identifier: Oklahoma #157370–1. Department of the Navy, Bureau of Construction and Repair. File Unit: Plans for Battleship USS Oklahoma (BB-37), 1919–1940.
USMC Historical Monograph, The Battle for Tarawa. Captain James R. Stockman, USMC, Historical Section, Division of Public Information, Headquarters US Marine Corps, Washington, DC; 1947.
Trotter M. Notes: Historical in so far as the Cabanatuan burials and disposition has been made, 24 October 1951, Mildred Trotter Papers, Special Collections of the Bernard Becker Medical Library. St. Louis, MO, Washington University; 1951.
Beckenbaugh L, Harris H Casualties of Cabanatuan Prisoner of War Camp #1 and the history of their burials. Archival Research Memo, Department of Defense Prisoner of War/Missing Personnel Office (DPMO), Washington, DC; 2005.
468 Graves Registration Service. RG 92 Records of the Office of the Quartermaster General, record group 92, entry 1894, Subseries 2, Box 31; 1948.
Edson SM. Extraction of DNA from skeletonized post-cranial remains: A discussion of protocols and testing modalities. J For Sci. 2019;64:1312–3.
Loreille OM, Diegoli TM, Irwin JA, Coble MD, Parsons TJ. High efficiency DNA extraction from bone by total demineralization. Forensic Sci Intl Genet. 2007;1:191–5.
Loreille OM, Parr RL, McGregor KA, Fitzpatrick CM, Lyon C, Yang DY, et al. Integrated DNA and fingerprint analyses in the identification of 60-year-old mummified human remains discovered on an Alaskan glacier. J Forensic Sci. 2010;55:813–8.
Edson SM, McMahon TP. Extraction of DNA from skeletal Remains. In: Goodwin W, editor. Forensic DNA typing protocols, methods in molecular biology, vol. 1420. New York, NY: Humana Press; 2016. p. 69–87.
Edson SM, Ross JP, Coble MD, Parsons TJ, Barritt SM. Naming the dead: Confronting the realities of the rapid identification of degraded skeletal remains. Forensic Sci Rev. 2004;16:63–90.
Gabriel M, Huffine E, Ryan J, Holland M, Parsons T. Improved mtDNA sequence analysis of forensic remains using a “mini-primer set” amplification strategy. J Forensic Sci. 2001;46:247–53.
Sturk KA, Coble MD, Barritt SM, Irwin JA. Evaluation of modified Yfiler™ amplification strategy for compromised samples. Croat Med J. 2009;50:228–38.
Irwin JA, Edson SM, Loreille O, Just RS, Barritt SM, Lee DA, et al. DNA identification of “Earthquake McGoon” 50 years postmortem. J Forensic Sci. 2007;52:1115–8.
Marshall C, Sturk-Andreaggi K, Daniels-Higginbotham J, Oliver RS, Barritt-Ross SM, McMahon TP. Performance evaluation of a mitogenome capture and Illumina sequencing protocol using non-probative, case-type skeletal samples: Implications for the use of a positive control in a next-generation sequencing procedure. Forensic Sci Intl Genet. 2017;31:198–206.
Prinz M, Carracedo A, Mayr WR, Morling N, Parsons TJ, Sajantila A, et al. DNA commission of the International Society for Forensic Genetics (ISFG): Recommendations regarding the role of forensic genetics for disaster victim identification (DVI). Forensic Sci Int Genet. 2007;1:3–12.
Pinhasi R, Fernandes D, Sirak K, Novak M, Connell S, Alpaslan-Roodenberg S, et al. Optimal ancient DNA yields from the inner ear part of the human petrous bone. PLoS One. 2015;10:e0129102.
Hansen HB, Damgaard PB, Margaryan A, Stenderup J, Lynnerup N, Willerslev E, et al. Comparing ancient DNA preservation in petrous bone and tooth cementum. PLoS One. 2017;12:e0170940.
Edson SM, McMahon TP. Testing of skeletonized human remains using GC/MS – Development of a personal environmental profile. Aust J Forensic Sci. 2019;51:S115–8.
Kuykendall JR, Bogdanffy MS. Efficiency of a DNA-histone crosslinking induced by saturated and unsaturated aldehydes in vitro. Mutation Res Letters. 1992;283:131–6.
Edson SM. Getting ahead: Extraction of DNA from skeletonized cranial mateirla and teeth. J For Sci. 2019. https://doi.org/10.1111/1556-4029.14123.
Mundorff A, Davoren JM. Examination of DNA yield rates for different skeletal elements at increasing post mortem intervals. Forensic Sci Intl Genet. 2014;8:55–63.
Acknowledgements
The author wishes to acknowledge the assistance of Stephanie Ah Sam and Alexander Christensen in the compilation of this data; Adrian Linacre, Timothy McMahon, and Greg Berg for commentary and editorial suggestions; and Suzanne Barritt-Ross and the scientists of the Past Accounting Section of AFDIL, without whom this study would not have been possible.
Funding
The author is an employee of their agency and otherwise received no monetary support for this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Ethical approval
While human materials were used in this study, the analysis presented herein is a reporting of the results of active casework. No personally identifiable information or genetic data is presented. Approval to report the testing results was granted by the author’s agency.
Disclaimer
The opinions or assertions presented are the private views of the author and should not be construed as official or as reflecting the views of the Department of Defense; the Defense Health Agency; the Armed Forces Medical Examiner System; or the Defense POW/MIA Accounting Agency.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Edson, S.M. The effect of chemical compromise on the recovery of DNA from skeletonized human remains: A study of three World War II era incidents recovered from tropical locations. Forensic Sci Med Pathol 15, 542–554 (2019). https://doi.org/10.1007/s12024-019-00179-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12024-019-00179-2