Abstract
Purpose
We aimed to evaluate the clinical efficacy and safety of lenvatinib in patients with advanced anaplastic thyroid cancer (ATC) in real-world practice.
Methods
This multicenter, retrospective cohort study included 14 patients with advanced ATC who received lenvatinib. We evaluated the response rate according to RECIST.
Results
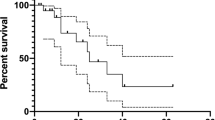
Ten patients had de novo ATC, and lenvatinib was used as a neoadjuvant treatment in eight patients. During a median follow-up of 6.7 months, patients received lenvatinib at a median dose of 13 mg daily. Overall, four patients (29%) showed partial response, nine (64%) had stable disease, and one (7%) had progressive disease. Tumor burden was reduced in 13 patients (93%), and the median best percent change from the baseline was −15.8%. The median progression-free survival and overall survival were 5.7 months (95% confidence interval [CI], 2.2–8.3) and 6.7 months (95% CI, 3.0–8.4), respectively. All patients experienced adverse events (AEs). Most AEs were manageable but two AEs—tracheal perforation, and pneumothorax and pneumomediastinum—were life-threatening. One patient underwent flap surgery for reconstruction of their tracheal perforation, and another died of pneumothorax and pneumomediastinum, which seemed to be related to lenvatinib.
Conclusions
In this multicenter real-world study, lenvatinib demonstrated limited clinical activity in advanced ATC. It effectively reduced the tumor burden but showed doubtful survival benefit. Although most AEs were manageable, one fatal AE was related to rapid tumor shrinkage. Further studies are needed to clarify the efficacy and optimal dose of lenvatinib in patients with advanced ATC.



Similar content being viewed by others
References
E. Molinaro, C. Romei, A. Biagini, E. Sabini, L. Agate, S. Mazzeo, G. Materazzi, S. Sellari-Franceschini, A. Ribechini, L. Torregrossa, F. Basolo, P. Vitti, R. Elisei, Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat. Rev. Endocrinol. 13(11), 644–660 (2017). https://doi.org/10.1038/nrendo.2017.76
A.V. Chintakuntlawar, R.L. Foote, J.L. Kasperbauer, K.C. Bible, Diagnosis and management of anaplastic thyroid cancer. Endocrinol. Metab. Clin. North Am. 48(1), 269–284 (2019). https://doi.org/10.1016/j.ecl.2018.10.010
R.C. Smallridge, J.A. Copland, Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin. Oncol. (R. Coll. Radiol.) 22(6), 486–497 (2010). https://doi.org/10.1016/j.clon.2010.03.013
R.C. Smallridge, Approach to the patient with anaplastic thyroid carcinoma. J. Clin. Endocrinol. Metab. 97(8), 2566–2572 (2012). https://doi.org/10.1210/jc.2012-1314
J. Chen, J.D. Tward, D.C. Shrieve, Y.J. Hitchcock, Surgery and radiotherapy improves survival in patients with anaplastic thyroid carcinoma: analysis of the surveillance, epidemiology, and end results 1983-2002. Am. J. Clin. Oncol. 31(5), 460–464 (2008). https://doi.org/10.1097/COC.0b013e31816a61f3
A.K. Dumke, T. Pelz, D. Vordermark, Long-term results of radiotherapy in anaplastic thyroid cancer. Radiat. Oncol. 9(1), 90 (2014). https://doi.org/10.1186/1748-717x-9-90
K. Shimaoka, D.A. Schoenfeld, W.D. DeWys, R.H. Creech, R. DeConti, A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer 56(9), 2155–2160 (1985). https://doi.org/10.1002/1097-0142(19851101)56:9<2155::aid-cncr2820560903>3.0.co;2-e
K.B. Ain, M.J. Egorin, P.A. DeSimone, Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Collaborative Anaplastic Thyroid Cancer Health Intervention Trials (CATCHIT) Group. Thyroid 10(7), 587–594 (2000). https://doi.org/10.1089/thy.2000.10.587
J.A. Sosa, R. Elisei, B. Jarzab, J. Balkissoon, S.P. Lu, C. Bal, S. Marur, A. Gramza, R.B. Yosef, B. Gitlitz, B.R. Haugen, F. Ondrey, C. Lu, S.M. Karandikar, F. Khuri, L. Licitra, S.C. Remick, Randomized safety and efficacy study of fosbretabulin with paclitaxel/carboplatin against anaplastic thyroid carcinoma. Thyroid 24(2), 232–240 (2014). https://doi.org/10.1089/thy.2013.0078
M. Tahara, N. Kiyota, T. Yamazaki, N. Chayahara, K. Nakano, L. Inagaki, K. Toda, T. Enokida, H. Minami, Y. Imamura, T. Sasaki, T. Suzuki, K. Fujino, C.E. Dutcus, S. Takahashi, Lenvatinib for anaplastic thyroid cancer. Front Oncol 7, 25 (2017). https://doi.org/10.3389/fonc.2017.00025
T. Fukuhara, R. Donishi, S. Koyama, N. Miyake, E. Matsuda, K. Fujiwara, H. Kitano, H. Takeuchi, Significant amelioration of tracheal stenosis following lenvatinib in a patient who has anaplastic thyroid carcinoma with bronchomediastinal infiltration: a case report. Case Rep. Oncol 10(1), 175–181 (2017). https://doi.org/10.1159/000457831
S. Koyama, N. Miyake, K. Fujiwara, T. Morisaki, T. Fukuhara, H. Kitano, H. Takeuchi, Lenvatinib for Anaplastic Thyroid Cancer and Lenvatinib-Induced Thyroid Dysfunction. Eur. Thyroid J. 7(3), 139–144 (2018). https://doi.org/10.1159/000485972
J.I. Ohkubo, A. Takahashi, S. Ikezaki, F. Takenaga, Y. Ohkubo, H. Suzuki, Anaplastic thyroid carcinoma treated with Lenvatinib. Kurume Med. J. 64(1.2), 29–33 (2018). https://doi.org/10.2739/kurumemedj.MS6406
K. Oishi, D. Takabatake, Y. Shibuya, Efficacy of lenvatinib in a patient with anaplastic thyroid cancer. Endocrinol. Diabetes Metab. Case Rep. 2017 (2017). https://doi.org/10.1530/edm-16-0136
M. Schlumberger, M. Tahara, L.J. Wirth, B. Robinson, M.S. Brose, R. Elisei, M.A. Habra, K. Newbold, M.H. Shah, A.O. Hoff, A.G. Gianoukakis, N. Kiyota, M.H. Taylor, S.B. Kim, M.K. Krzyzanowska, C.E. Dutcus, B. de las Heras, J. Zhu, S.I. Sherman, Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 372(7), 621–630 (2015). https://doi.org/10.1056/NEJMoa1406470
P. Therasse, S.G. Arbuck, E.A. Eisenhauer, J. Wanders, R.S. Kaplan, L. Rubinstein, J. Verweij, M. Van Glabbeke, A.T. van Oosterom, M.C. Christian, S.G. Gwyther, New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 92(3), 205–216 (2000). https://doi.org/10.1093/jnci/92.3.205
A.C. Dubbelman, H. Rosing, C. Nijenhuis, A.D. Huitema, M. Mergui-Roelvink, A. Gupta, D. Verbel, G. Thompson, R. Shumaker, J.H. Schellens, J.H. Beijnen, Pharmacokinetics and excretion of (14)C-lenvatinib in patients with advanced solid tumors or lymphomas. Invest. New Drugs 33(1), 233–240 (2015). https://doi.org/10.1007/s10637-014-0181-7
K. Yamada, N. Yamamoto, Y. Yamada, H. Nokihara, Y. Fujiwara, T. Hirata, F. Koizumi, K. Nishio, N. Koyama, T. Tamura, Phase I dose-escalation study and biomarker analysis of E7080 in patients with advanced solid tumors. Clin. Cancer Res. 17(8), 2528–2537 (2011). https://doi.org/10.1158/1078-0432.Ccr-10-2638
M. Tahara, M.S. Brose, L.J. Wirth, T. Suzuki, H. Miyagishi, K. Fujino, C.E. Dutcus, A. Gianoukakis, Impact of dose interruption on the efficacy of lenvatinib in a phase 3 study in patients with radioiodine-refractory differentiated thyroid cancer. Eur. J. Cancer 106, 61–68 (2019). https://doi.org/10.1016/j.ejca.2018.10.002
E. Song, M. Kim, E.Y. Kim, B.H. Kim, D.Y. Shin, H.C. Kang, B.C. Ahn, K.B. Kim, Y.K. Shong, M.J. Jeon, D.J. Lim, Lenvatinib for radioactive iodine-refractory differentiated thyroid carcinoma and candidate biomarkers associated with survival: a multicenter study in Korea. Thyroid (2020). https://doi.org/10.1089/thy.2019.0476
P.C. Iyer, R. Dadu, R. Ferrarotto, N.L. Busaidy, M.A. Habra, M. Zafereo, N. Gross, K.R. Hess, M. Gule-Monroe, M.D. Williams, M.E. Cabanillas, Real-world experience with targeted therapy for the treatment of anaplastic thyroid Carcinoma. Thyroid 28(1), 79–87 (2018). https://doi.org/10.1089/thy.2017.0285
E. Kebebew, F.S. Greenspan, O.H. Clark, K.A. Woeber, A. McMillan, Anaplastic thyroid carcinoma. Treat. Out. Prog. Factors. Cancer 103(7), 1330–1335 (2005). https://doi.org/10.1002/cncr.20936
V. Subbiah, R.J. Kreitman, Z.A. Wainberg, J.Y. Cho, J.H.M. Schellens, J.C. Soria, P.Y. Wen, C. Zielinski, M.E. Cabanillas, G. Urbanowitz, B. Mookerjee, D. Wang, F. Rangwala, B. Keam, Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J. Clin. Oncol. 36(1), 7–13 (2018). https://doi.org/10.1200/jco.2017.73.6785
J.R. Wang, M.E. Zafereo, R. Dadu, R. Ferrarotto, N.L. Busaidy, C. Lu, S. Ahmed, M.K. Gule-Monroe, M.D. Williams, E.M. Sturgis, R.P. Goepfert, N.D. Gross, S.Y. Lai, G.B. Gunn, J. Phan, D.I. Rosenthal, C.D. Fuller, W.H. Morrison, P. Iyer, M.E. Cabanillas, Complete surgical resection following neoadjuvant dabrafenib plus trametinib in BRAF(V600E)-mutated anaplastic thyroid carcinoma. Thyroid 29(8), 1036–1043 (2019). https://doi.org/10.1089/thy.2019.0133
Funding
This research did not receive any specific grant from any funding agency in public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study protocol was reviewed and approved by the Institutional Review Board of each participating institution.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kim, M., Ahn, J., Song, D.E. et al. Real-world experience of lenvatinib in patients with advanced anaplastic thyroid cancer. Endocrine 71, 427–433 (2021). https://doi.org/10.1007/s12020-020-02425-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02425-y