Abstract
Purpose
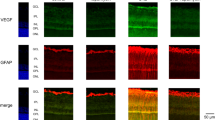
In the earliest stages of diabetic retinopathy (DR), a dysfunction of Müller cells, characterized by high levels of glial fibrillary acidic protein (GFAP), and aquaporins (AQP), has been observed. Although chronic hyperglycemia causes the activation of Müller cells, the effect of glycemic fluctuations is yet unknown. The aim of the study was to analyze the impact of glucose variability on rat retinal Müller cells (rMC-1) adapted to either normal (5 mM) or high (25 mM) glucose levels.
Methods
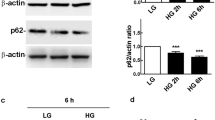
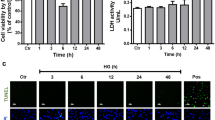
rMC-1 were cultured in a medium containing either 5 mM (N cells) or 25 mM of glucose (H cells) and then incubated for 96 h in a medium containing (a) low glucose (either 1–3 or 5 mM), (b) basal glucose (either 5 or 25 mM), (c) high glucose (either 25 or 45 mM), (d) basal and high glucose alternated every 24 h; (e) low- and high glucose alternated every 24 h; (f) basal glucose with episodes of low glucose for 30 min twice a day. Müller cells activation was evaluated by measuring the levels of GFAP, AQP4, and phospho-active extracellular signal-regulated kinase (pERK).
Results
Under both basal and high glucose concentrations rMC-1 were viable, but their response to glucose excursions was different. In N cells kept under normal (5 mM) glucose, a significant glial activation was measured not only in response to constant high glucose but also to alternating low/high glucose. In H cells, adapted to 25 mM glucose, a significant response was observed only after exposition to a lower (5 mM) glucose concentration.
Conclusion
Our results highlight Müller cells activation in response to glucose variability and a different susceptibility depending on the basal glucose conditions.





Similar content being viewed by others
Abbreviations
- DR:
-
diabetic retinopathy
- GFAP:
-
glial fibrillary acidic protein
- AQP:
-
aquaporin
- rMC-1:
-
retinal Müller cells
- pERK:
-
phospho-active extracellular signal-regulated kinase
- RGC:
-
retinal ganglion cells
- N cells:
-
5 mM (1 g/L) glucose
- H cells:
-
25 mM (4.5 g/L) glucose
- GLUT1:
-
glucose transporter-1
- GLP-1RA:
-
glucagon-like peptide 1 receptor agonists
References
R. Lee, T.Y. Wong, C. Sabanayagam, Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2, 17 (2015)
S.F. Abcouwer, T.W. Gardner, Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann. N. Y. Acad. Sci. 1311, 174–190 (2014).
D.S. Ng, P.P. Chiang, G. Tan, C.G. Cheung et al. Retinal ganglion cell neuronal damage in diabetes and diabetic retinopathy. Clin. Exp. Ophthalmol. 44(4), 243–50 (2016)
A. Bringmann, P. Wiedemann, Müller Glial Cells in Retinal Disease. Ophthalmologica 227, 1–19 (2012)
B.A. Coughlin, D.J. Feenstra, S. Mohr, Müller cells and diabetic retinopathy. Vis. Res. 139, 93–100 (2017)
S. Vujosevic, A. Micera, S. Bini, M. Berton, G. Esposito, E. Midena, Aqueous Humor Biomarkers of Müller Cell Activation in Diabetic Eyes. Invest. Ophthalmol. Vis. Sci. 56, 3913–3918 (2015).
Y. Jiang, S.K. Biswas, J.J. Steinle, Serine 307 on insulin receptor substrate 1 is required for SOCS3 and TNF-α signaling in the rMC-1 cell line. Mol. Vis. 20, 1463–1470 (2014)
B. Cui, J.H. Sun, F.F. Xiang, L. Liu, W.J. Li, Aquaporin 4 knockdown exacerbates streptozotocin-induced diabetic retinopathy through aggravating inflammatory response. Exp. Eye Res. 98, 37–43 (2012)
M.C. MacGregorl, L.R. Rosecan, A.M. Laties, F.M. Matschinskye, Altered Retinal Metabolism in Diabetes. J. Biol. Chem. 261(9), 4046–4051 (1986)
Y. Ueda, A. Inui, Y. Mifune et al. The effects of high glucose condition on rat tenocytes in vitro and rat Achilles tendon in vivo. Bone Joint Res. 7(5), 362–372 (2018)
Y. Huang, Z.G. Xiong, Choosing an appropriate glucose concentration according to different cell types and experimental purposes is very important. Cell Stress Chaperons 20, 1–2 (2015)
V.P. Sarthy, S.J. Brodjian, K. Dutt, B.N. Kennedy, R.P. French, J.W. Crabb, Establishment and characterization of a retinal Müller cell line. Invest. Ophthalmol. Vis. Sci. 39(1), 212–6 (1998)
L. Quagliaro, L. Piconi, R. Assaloni, L. Martinelli, E. Motz, A. Ceriello, Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 52, 2795–2804 (2003)
J. Sun, Y. Xu, S. Sun, Y. Sun, X. Wang, Intermittent high glucose enhances cell proliferation and VEGF expression in retinal endothelial cells: the role of mitochondrial reactive oxygen species. Mol. Cell Biochem. 343, 27–35 (2010)
J. Sun, Y. Xu, H. Deng, S. Sun, Z. Dai, Y. Sun, Intermittent high glucose exacerbates the aberrant production of adiponectin and resistin through mitochondrial superoxide overproduction in adipocytes. J. Molen. 44, 179–185 (2010)
L.Q. Sun, Y.Y. Chen, X. Wang et al. The protective effect of alpha lipoic acid on Schwann cells exposed to constant or intermittent high glucose. Biochem. Pharmacol. 84, 961–973 (2012)
K.D. Kohnert, E.J. Freyse, E. Salzsieder, Glycaemic variability and pancreatic β-cell dysfunction. Curr. Diabetes Rev. 8, 345–354 (2012)
K.E. Trueblood, S. Mohr, G.R. Dubyak, Purinergic regulation of high-glucose-induced caspase-1 activation in the rat retinal Müller cell line rMC-1. Am. J. Physiol. Cell Physiol. 301(5), C1213–23 (2011)
Y. Zhong, J. Li, Y. Chen, J.J. Wang, R. Ratan, S.X. Zhang, Activation of endoplasmic reticulum stress by hyperglycemia is essential for Müller cell-derived inflammatory cytokine production in diabetes. Diabetes 61(2), 492–504 (2012)
E.A. Nagelhus, O.P. Ottersen, Physiological roles of aquaporin-4 in brain. Physiol. Rev. 93(4), 1543–62 (2013). https://doi.org/10.1152/physrev.00011.2013
F. Picconi, M. Parravano, D. Ylli et al. Retinal neurodegeneration in patients with type 1 diabetes mellitus: the role of glycemic variability. Acta. Diabetol 54(5), 489–497 (2017)
X. Ye, H. Ren, M. Zhang, Z. Sun, A.C. Jiang, G. Xu, ERK1/2 signaling pathway in the release of VEGF from Müller cells in diabetes. Invest. Ophthalmol. Vis. Sci. 53(7), 3481–3489 (2012)
A. Matteucci, L. Gaddini, M. Villa et al. Neuroprotection by rat Müller glia against high glucose-induced neurodegeneration through a mechanism involving ERK1/2 activation. Exp. Eye Res. 125, 20–9 (2014)
G. Groeger, F. Doonan, T.G. Cotter, M. Donovan, Reactive oxygen species regulate prosurvival ERK1/2 signaling and bFGF expression in gliosis within the retina. Invest. Ophthalmol. Vis. Sci. 53(10), 6645–54 (2012)
A. Nose, Y. Mori, Y. Uchiyama-Tanaka et al. Regulation of glucose transporter (GLUT1) gene expression by angiotensin II in mesangial cells: involvement of HB-EGF and EGF receptor transactivation. Hypertens. Res. 26(1), 67–73 (2003)
A.K. Kumagai, Glucose transport in brain and retina: implications in the management and complications of diabetes. Diabetes Metab. Res. Rev. 15(4), 261–73 (1999)
F. Moruno, E. Pérez-Jiménez, E. Knecht, Regulation of autophagy by glucose in mammalian cells. Cells 1(3), 372–395 (2012)
J. Wang, M.W. Whiteman, H. Lian et al. ERK1/2 plays a role in the activation of autophagy. J. Biol. Chem. 284(32), 21412–24 (2009)
C.H. Wong, K.B. Iskandar, S.K. Yadav, J.L. Hirpara, T. Loh, S. Pervaiz, Simultaneous induction of non-canonical autophagy and apoptosis in cancer cells by ROS-dependent ERK and JNK activation. PLoS ONE 5(4), e9996 (2010)
W. Zhang, J. Song, Y. Zhang et al. Intermittent high glucose-induced oxidative stress modulates retinal pigmented epithelial cell autophagy and promotes cell survival via increased HMGB1.BMC. Ophthalmol. 18, 192 (2018)
L.E. Johnson, M. Larsen, M.T. Perez, Retinal adaptation to changing glycemic levels in a rat model of type 2 diabetes. PLoS ONE 8, 55456 (2013)
S.P. Marso, S.C. Bain, A. Consoli et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016)
L. Varadhan, T. Humphreys, C. Hariman, A.B. Walker, G.I. Varughese, GLP-1 agonist treatment: implications for diabetic retinopathy screening. Diabetes Res. Clin. Pract. 94, 68–71 (2011)
L. Varadhan, T. Humphreys, A.B. Walker, G.I. Varughese, The impact of improved glycaemic control with GLP-1 receptor agonist therapy on diabetic retinopathy. Diabetes Res. Clin. Pract. 103, 37–39 (2014)
Acknowledgements
The research for this paper was partially supported by the Italian Ministry of Health and Fondazione Roma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Fabiana Picconi, Mariacristina Parravano
Rights and permissions
About this article
Cite this article
Picconi, F., Parravano, M., Sciarretta, F. et al. Activation of retinal Müller cells in response to glucose variability. Endocrine 65, 542–549 (2019). https://doi.org/10.1007/s12020-019-02017-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02017-5