Abstract
Purpose
Hyperprolactinemia (HPRL) has been reported in many autoimmune diseases. However, the serum autoantibody profile and peripheral B-cell subset distribution in women with HPRL are largely unknown. The current study aimed to investigate the autoantibody prevalence and cytokine levels as well as to further explore the B-cell subset distribution in women with HPRL.
Methods
Sera from 202 women with HPRL and 97 healthy women were included in this study. All sera were examined for prolactin (PRL), anti-nuclear antibody (ANA), rheumatoid factor, anticardiolipin (ACL), immunoglobulin G, immunoglobulin M, complement 3, complement 4, interleukin 4 (IL-4) and interleukin 6 (IL-6). Peripheral blood was collected from 22 women with HPRL and 19 healthy women, and B-cell subsets were measured by flow cytometry.
Results
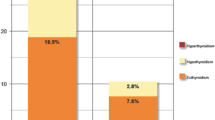
At least one autoantibody was found in 47 out of 202 women with HPRL compared with 9 of 97 healthy women (p < 0.001). The levels of IL-4 (p < 0.0001) and IL-6 (p < 0.0001) were significantly higher in women with HPRL than in healthy women. The percentages of naive IgD+IgM− B cells (BND cells, p < 0.0001), antibody-secreting cells (p = 0.007) and unswitched memory B cells (p = 0.004) among the total B cells from HPRL women were significantly higher than those from healthy women.
Conclusions
Women with HPRL had a higher prevalence of autoantibodies, higher serum levels of IL-4 and IL-6, and more BND cells, antibody-secreting B cells and unswitched memory B cells than healthy women. These data imply that a high level of PRL is associated with autoimmune diseases.



Similar content being viewed by others
References
G. Zandman-Goddard, E. Peeva, Y. Shoenfeld, Gender and autoimmunity. Autoimmun. Rev. 6(6), 366–372 (2007). https://doi.org/10.1016/j.autrev.2006.10.001
E. Ortona, M. Pierdominici, A. Maselli, C. Veroni, F. Aloisi, Y. Shoenfeld, Sex-based differences in autoimmune diseases. Ann. Ist. Super. Sanita 52(2), 205–212 (2016). https://doi.org/10.4415/ann_16_02_12
L.Y. Yu-Lee, Prolactin modulation of immune and inflammatory responses. Recent Prog. Horm. Res. 57, 435–455 (2002)
N. Ben-Jonathan, J.L. Mershon, D.L. Allen, R.W. Steinmetz, Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr. Rev. 17(6), 639–669 (1996). https://doi.org/10.1210/edrv-17-6-639
G. Recalde, T. Moreno-Sosa, F. Yudica, C.A. Quintero, M.B. Sanchez, G.A. Jahn, A.M. Kalergis, J.P. Mackern-Oberti, Contribution of sex steroids and prolactin to the modulation of T and B cells during autoimmunity. Autoimmun. Rev. 17(5), 504–512 (2018). https://doi.org/10.1016/j.autrev.2018.03.006
S. Shelly, M. Boaz, H. Orbach, Prolactin and autoimmunity. Autoimmun. Rev. 11(6-7), A465–A470 (2012). https://doi.org/10.1016/j.autrev.2011.11.009
V.V. Borba, G. Zandman-Goddard, Y. Shoenfeld, Prolactin and autoimmunity. Front. Immunol. 9, 73 (2018). https://doi.org/10.3389/fimmu.2018.00073
S. Melmed, F.F. Casanueva, A.R. Hoffman, D.L. Kleinberg, V.M. Montori, J.A. Schlechte, J.A. Wass, Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96(2), 273–288 (2011). https://doi.org/10.1210/jc.2010-1692
S.C. Dogansen, O.S. Selcukbiricik, B.E. Bilir, S. Yarman, The higher incidence of autoimmune thyroid disease in prolactinomas than in somatotrophinomas. Growth Horm. IGF Res. 29, 45–49 (2016). https://doi.org/10.1016/j.ghir.2016.04.004
A. Elenkova, C.I. capital A, C.G. capital Ka, C. Natchev capital Ie, R. Ivanova, C.R. capital Ka, S. Vandeva, D. Tcharaktchiev, S. Zacharieva, Autoimmune hypothyroidism is three times more frequent in female prolactinoma patients compared to healthy women: data from a cross-sectional case-control study. Endocrine 57(3), 486–493 (2017). https://doi.org/10.1007/s12020-017-1372-8
Z.D. Hu, A.M. Deng, Autoantibodies in pre-clinical autoimmune disease. Clin. Chim. Acta 437, 14–18 (2014). https://doi.org/10.1016/j.cca.2014.06.015
D. Buskila, M. Berezin, H. Gur, H.C. Lin, I. Alosachie, J.W. Terryberry, N. Barka, B. Shen, J.B. Peter, Y. Shoenfeld, Autoantibody profile in the sera of women with hyperprolactinemia. J. Autoimmun. 8(3), 415–424 (1995). https://doi.org/10.1006/jaut.1995.0033
I. Krause, Z. Blumenfeld, M. Malchinsky, S. Cohen, M. Blank, A. Eldor, B. Weksler, K. Schweitzer, Y. Shoenfeld, Anti-endothelial cell antibodies in the sera of hyperprolactinemic women. Lupus 7(6), 377–382 (1998). https://doi.org/10.1191/096120398678920316
Kalsi, A.K., Halder, A., Jain, M., Chaturvedi, P.K., Mathew, M., Sharma, J.B., Association of raised levels of IL-4 and anti-TPO with hyperprolactinemia. Am. J. Reprod. Immunol., e13085 (2019). https://doi.org/10.1111/aji.13085
M.W. Tang, S. Garcia, D.M. Gerlag, P.P. Tak, K.A. Reedquist, Insight into the endocrine system and the immune system: a review of the inflammatory role of prolactin in rheumatoid arthritis and psoriatic arthritis. Front. Immunol. 8, 720 (2017). https://doi.org/10.3389/fimmu.2017.00720
N.T. Ashley, G.E. Demas, Neuroendocrine-immune circuits, phenotypes, and interactions. Horm. Behav. 87, 25–34 (2017). https://doi.org/10.1016/j.yhbeh.2016.10.004
A.M. Jacobi, W. Rohde, H.D. Volk, T. Dorner, G.R. Burmester, F. Hiepe, Prolactin enhances the in vitro production of IgG in peripheral blood mononuclear cells from patients with systemic lupus erythematosus but not from healthy controls. Ann. Rheum. Dis. 60(3), 242–247 (2001)
E. Peeva, D. Michael, J. Cleary, J. Rice, X. Chen, B. Diamond, Prolactin modulates the naive B cell repertoire. J. Clin. Invest. 111(2), 275–283 (2003). https://doi.org/10.1172/jci16530
Y.H. Lee, S.C. Bae, G.G. Song, Meta-analysis of associations between functional prolactin -1149 G/T polymorphism and susceptibility to rheumatoid arthritis and systemic lupus erythematosus. Clin. Rheumatol. 34(4), 683–690 (2015). https://doi.org/10.1007/s10067-015-2904-3
Y.H. Tseng, M.A. Kessler, L.A. Schuler, Regulation of interleukin (IL)-1alpha, IL-1beta, and IL-6 expression by growth hormone and prolactin in bovine thymic stromal cells. Mol. Cell Endocrinol. 128(1-2), 117–127 (1997)
P. Scarfogliero, M. Vitiello, M. Galdiero, F. Brancaccio, L. Sommese, Prolactin regulates IL-1 alpha, IFN-gamma and IL-4 release from mouse splenocytes stimulated with some staphylococcal and streptococcal toxins. New Microbiol. 19(4), 301–308 (1996)
L. Yang, Y. Hu, X. Li, J. Zhao, Y. Hou, Prolactin modulates the functions of murine spleen CD11c-positive dendritic cells. Int. Immunopharmacol. 6(9), 1478–1486 (2006). https://doi.org/10.1016/j.intimp.2006.05.001
J.E. Ochoa-Amaya, E.K. Hamasato, C.N. Tobaruela, N. Queiroz-Hazarbassanov, J.A. Anselmo Franci, J. Palermo-Neto, F.R. Greiffo, A.A. de Britto, R.P. Vieira, A.P. Ligeiro de Oliveira, O. Massoco Cde, L.F. Felicio Short-term hyperprolactinemia decreases allergic inflammatory response of the lungs. Life. Sci. 142, 66–75 (2015). https://doi.org/10.1016/j.lfs.2015.10.016
D. Xu, L. Lin, X. Lin, Z. Huang, Z. Lei, Immunoregulation of autocrine prolactin: suppressing the expression of costimulatory molecules and cytokines in T lymphocytes by prolactin receptor knockdown. Cell Immunol. 263(1), 71–78 (2010). https://doi.org/10.1016/j.cellimm.2010.02.018
C.M. Tipton, C.F. Fucile, J. Darce, A. Chida, T. Ichikawa, I. Gregoretti, S. Schieferl, J. Hom, S. Jenks, R.J. Feldman, R. Mehr, C. Wei, F.E. Lee, W.C. Cheung, A.F. Rosenberg, I. Sanz, Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat. Immunol. 16(7), 755–765 (2015). https://doi.org/10.1038/ni.3175
Germar, K., Fehres, C.M., Scherer, H.U., van Uden, N., Pollastro, S., Yeremenko, N., Hansson, M., Kerkman, P.F., van der Voort, E.I.H., Reed, E., Maassen, H., Kwakkenbos, M.J., Bakker, A.Q., Klareskog, L., Malmstrom, V., de Vries, N., Toes, R.E.M., Lundberg, K., Spits, H., Baeten, D.L., Generation and characterization of anti-citrullinated protein antibody-producing B cell clones from rheumatoid arthritis patients. Arthritis Rheumatol. (2018). https://doi.org/10.1002/art.40739
A.R. Buckley, Prolactin, a lymphocyte growth and survival factor. Lupus 10(10), 684–690 (2001). https://doi.org/10.1191/096120301717164912
C.A. Hunter, S.A. Jones, IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 16(5), 448–457 (2015). https://doi.org/10.1038/ni.3153
J.A. Duty, P. Szodoray, N.Y. Zheng, K.A. Koelsch, Q. Zhang, M. Swiatkowski, M. Mathias, L. Garman, C. Helms, B. Nakken, K. Smith, A.D. Farris, P.C. Wilson, Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J. Exp. Med. 206(1), 139–151 (2009). https://doi.org/10.1084/jem.20080611
Szodoray, P., Stanford, S.M., Molberg, O., Munthe, L.A., Bottini, N., Nakken, B., T-helper signals restore B-cell receptor signaling in autoreactive anergic B cells by upregulating CD45 phosphatase activity. J. Allergy Clin. Immunol. (2016). https://doi.org/10.1016/j.jaci.2016.01.035
Smith, M.J., Rihanek, M., Coleman, B.M., Gottlieb, P.A., Sarapura, V.D., Cambier, J.C., Activation of thyroid antigen-reactive B cells in recent onset autoimmune thyroid disease patients. J. Autoimmun. (2017). https://doi.org/10.1016/j.jaut.2017.12.001
Smith, M.J., Packard, T.A., O’Neill, S.K., Henry Dunand, C.J., Huang, M., Fitzgerald-Miller, L., Stowell, D., Hinman, R.M., Wilson, P.C., Gottlieb, P.A., Cambier, J.C., Loss of anergic B cells in pre-diabetic and new onset T1D patients. Diabetes (2014). https://doi.org/10.2337/db13-1798
C. Wei, S. Jenks, I. Sanz, Polychromatic flow cytometry in evaluating rheumatic disease patients. Arthritis Res. Ther. 17, 46 (2015). https://doi.org/10.1186/s13075-015-0561-1
A. Shimatsu, N. Hattori, Macroprolactinemia: diagnostic, clinical, and pathogenic significance. Clin. Dev. Immunol. 2012, 167132 (2012). https://doi.org/10.1155/2012/167132
Acknowledgements
We thank all participating subjects who provided the blood samples and clinical information necessary for this study. We thank Dr. Fei Ye from the Department of Endocrinology, Changzheng Hospital, and Dr. Hu Peng from the Department of Head and Neck Surgery, Changzheng Hospital, for clinical medical history collection.
Funding
This study was funded by the National Natural Science Foundation of China (Grant 81430031, 81302578 and 81501401), the Major State Basic Research Development Program of China (973 Program, 2014CB541800) and the Pujiang Rheumatologist Training Program (SPROG201703).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All the recruited subjects who participated in the study provided informed consent. The study was reviewed and approved by the Medical Ethics Committee of Shanghai Changzheng Hospital, Second Military Medical University.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contribute equally: Yaoyang Liu, Zhiguo Zhang, Qianmei Jin
Rights and permissions
About this article
Cite this article
Liu, Y., Zhang, Z., Jin, Q. et al. Hyperprolactinemia is associated with a high prevalence of serum autoantibodies, high levels of inflammatory cytokines and an abnormal distribution of peripheral B-cell subsets. Endocrine 64, 648–656 (2019). https://doi.org/10.1007/s12020-019-01896-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-01896-y