Abstract
Purpose
Present study aims to explore the pathophysiological role of microRNA (miR)-29a in the process of obesity-related cardiomyopathy in human subjects and mice.
Methods
The expression level of circulating exosomal miR-29a was measured in 37 lean and 30 obese human subjects, and correlated with cardiac parameters. The effects of miR-29a on mitochondrial activity and cardiac function were investigated by treatment of miR-29a sponge in primary mouse cardiomyocytes and diet-induced obesity-related cardiomyopathy in mice.
Results
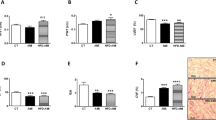
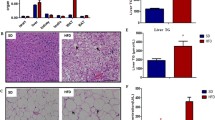
The increased circulating miR-29a level was closely associated with impaired human cardiac function, including ejection fraction (r = −0.2663, p < 0.05) and NT-proBNP levels (r = 0.4270, p < 0.001). Exosomes from obese human plasma mediated cardiomyocyte mitochondrial inactivity, but pre-treatment with miR-29a sponge attenuated the exosomal miR-29a-induced reduction of ATP production (p < 0.001), basal oxygen consumption (p < 0.01) and mitochondrial complex I activity (p < 0.01). In vivo mouse study, high fat diet damaged cardiac function, normal structure, and mitochondrial activity, whereas miR-29a sponge improved the cardiac status.
Conclusions
Present study uncovered the correlation between circulating miR-29a and cardiac parameters in human subjects, and provided solid evidence of the therapeutic application of miR-29a sponge in combating obesity-mediated cardiac dysfunction.



Similar content being viewed by others

References
H.B. Hubert, M. Feinleib, P.M. McNamara, W.P. Castelli, Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 67(5), 968–977 (1983)
L.F. Van Gaal, I.L. Mertens, C.E. De Block, Mechanisms linking obesity with cardiovascular disease. Nature 444(7121), 875–880 (2006). https://doi.org/10.1038/nature05487
T.B. Horwich, G.C. Fonarow, M.A. Hamilton, W.R. MacLellan, M.A. Woo, J.H. Tillisch, The relationship between obesity and mortality in patients with heart failure. J. Am. Coll. Cardiol. 38(3), 789–795 (2001)
G. O’Brien, Obesity and the risk of heart failure. N. Eng. J. Med. 347(23), 1887–1889 (2002). https://doi.org/10.1056/NEJM200212053472314
A.B. Gustafsson, R.A. Gottlieb, Heart mitochondria: gates of life and death. Cardiovasc. Res. 77(2), 334–343 (2008). https://doi.org/10.1093/cvr/cvm005
M. Frisard, E. Ravussin, Energy metabolism and oxidative stress: impact on the metabolic syndrome and the aging process. Endocrine 29(1), 27–32 (2006). https://doi.org/10.1385/ENDO:29:1:27
J. Marin-Garcia, M.J. Goldenthal, G.W. Moe, Abnormal cardiac and skeletal muscle mitochondrial function in pacing-induced cardiac failure. Cardiovasc. Res. 52(1), 103–110 (2001)
J. Hirst, Mitochondrial complex I. Annu. Rev. Biochem. 82, 551–575 (2013). https://doi.org/10.1146/annurev-biochem-070511-103700
J.G. Duncan, B.N. Finck, The PPARalpha-PGC-1alpha axis controls cardiac energy metabolism in healthy and diseased myocardium. Ppar. Res. 2008, 253817 (2008). https://doi.org/10.1155/2008/253817
K. Drosatos, P.C. Schulze, Cardiac lipotoxicity: molecular pathways and therapeutic implications. Curr. Heart Fail. Rep. 10(2), 109–121 (2013). https://doi.org/10.1007/s11897-013-0133-0
I. Cundrle, P. Suk, V. Sramek, Z. Lacinova, M. Haluzik, Circadian leptin concentration changes in critically ill heart failure patients-preliminary results. Physiol. Res. 67(3), 505–508 (2018)
M.C. Borges, D.A. Lawlor, C. de Oliveira, J. White, B.L. Horta, A.J. Barros, Role of adiponectin in coronary heart disease risk: a mendelian randomization study. Circ. Res. 119(3), 491–499 (2016). https://doi.org/10.1161/CIRCRESAHA.116.308716
K.M. Choi, J.S. Lee, E.J. Kim, S.H. Baik, H.S. Seo, D.S. Choi, D.J. Oh, C.G. Park, Implication of lipocalin-2 and visfatin levels in patients with coronary heart disease. Eur. J. Endocrinol. 158(2), 203–207 (2008). https://doi.org/10.1530/EJE-07-0633
R. Shibata, N. Ouchi, M. Ito, S. Kihara, I. Shiojima, D.R. Pimentel, M. Kumada, K. Sato, S. Schiekofer, K. Ohashi, T. Funahashi, W.S. Colucci, K. Walsh, Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat. Med. 10(12), 1384–1389 (2004). https://doi.org/10.1038/nm1137
N.C. Lau, L.P. Lim, E.G. Weinstein, D.P. Bartel, An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 294(5543), 858–862 (2001). https://doi.org/10.1126/science.1065062
R.A. Boon, K. Iekushi, S. Lechner, T. Seeger, A. Fischer, S. Heydt, D. Kaluza, K. Treguer, G. Carmona, A. Bonauer, A.J. Horrevoets, N. Didier, Z. Girmatsion, P. Biliczki, J.R. Ehrlich, H.A. Katus, O.J. Muller, M. Potente, A.M. Zeiher, H. Hermeking, S. Dimmeler, MicroRNA-34a regulates cardiac ageing and function. Nature 495(7439), 107–110 (2013). https://doi.org/10.1038/nature11919
M. Kelly, R.D. Bagnall, R.E. Peverill, L. Donelan, L. Corben, M.B. Delatycki, C. Semsarian, A polymorphic miR-155 binding site in AGTR1 is associated with cardiac hypertrophy in Friedreich ataxia. J. Mol. Cell. Cardiol. 51(5), 848–854 (2011). https://doi.org/10.1016/j.yjmcc.2011.07.001
F. Wang, S.J. Zhang, X. Yao, D.M. Tian, K.Q. Zhang, D.M. She, F.F. Guo, Q.W. Zhai, H. Ying, Y. Xue, Circulating microRNA-1a is a biomarker of Graves’ disease patients with atrial fibrillation. Endocrine 57(1), 125–137 (2017). https://doi.org/10.1007/s12020-017-1331-4
R. Roncarati, C. Viviani Anselmi, M.A. Losi, L. Papa, E. Cavarretta, P. Da Costa Martins, C. Contaldi, G. Saccani Jotti, A. Franzone, L. Galastri, M.V. Latronico, M. Imbriaco, G. Esposito, L. De Windt, S. Betocchi, G. Condorelli, Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 63(9), 920–927 (2014). https://doi.org/10.1016/j.jacc.2013.09.041
Y. Ye, Z. Hu, Y. Lin, C. Zhang, J.R. Perez-Polo, Downregulation of microRNA-29 by antisense inhibitors and a PPAR-gamma agonist protects against myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 87(3), 535–544 (2010). https://doi.org/10.1093/cvr/cvq053
A.A. Derda, S. Thum, J.M. Lorenzen, U. Bavendiek, J. Heineke, B. Keyser, M. Stuhrmann, R.C. Givens, P.J. Kennel, P.C. Schulze, J.D. Widder, J. Bauersachs, T. Thum, Blood-based microRNA signatures differentiate various forms of cardiac hypertrophy. Int. J. Cardiol. 196, 115–122 (2015). https://doi.org/10.1016/j.ijcard.2015.05.185
L. Fang, A.H. Ellims, X.L. Moore, D.A. White, A.J. Taylor, J. Chin-Dusting, A.M. Dart, Circulating microRNAs as biomarkers for diffuse myocardial fibrosis in patients with hypertrophic cardiomyopathy. J. Transl. Med. 13, 314 (2015). https://doi.org/10.1186/s12967-015-0672-0
L. Wang, X. Niu, J. Hu, H. Xing, M. Sun, J. Wang, Q. Jian, H. Yang, After myocardial ischemia-reperfusion, miR-29a, and Let7 could affect apoptosis through regulating IGF-1. Biomed. Res. Int. 2015, 245412 (2015). https://doi.org/10.1155/2015/245412
Y. Zhang, X.R. Huang, L.H. Wei, A.C. Chung, C.M. Yu, H.Y. Lan, miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-beta/Smad3 signaling. Mol. Ther. 22(5), 974–985 (2014). https://doi.org/10.1038/mt.2014.25
X. Cao, J. Wang, Z. Wang, J. Du, X. Yuan, W. Huang, J. Meng, H. Gu, Y. Nie, B. Ji, S. Hu, Z. Zheng, MicroRNA profiling during rat ventricular maturation: a role for miR-29a in regulating cardiomyocyte cell cycle re-entry. FEBS Lett. 587(10), 1548–1555 (2013). https://doi.org/10.1016/j.febslet.2013.01.075
W.M. Yang, H.J. Jeong, S.Y. Park, W. Lee, Induction of miR-29a by saturated fatty acids impairs insulin signaling and glucose uptake through translational repression of IRS-1 in myocytes. FEBS Lett. 588(13), 2170–2176 (2014). https://doi.org/10.1016/j.febslet.2014.05.011
J. Dooley, J.E. Garcia-Perez, J. Sreenivasan, S.M. Schlenner, R. Vangoitsenhoven, A.S. Papadopoulou, L. Tian, S. Schonefeldt, L. Serneels, C. Deroose, K.A. Staats, B. Van der Schueren, B. De Strooper, O.P. Mc Guinness, C. Mathieu, A. Liston, The microRNA-29 family dictates the balance between homeostatic and pathological glucose handling in diabetes and obesity. Diabetes 65(1), 53–61 (2016). https://doi.org/10.2337/db15-0770
Y. Pan, Y. Wang, Y. Zhao, K. Peng, W. Li, Y. Wang, J. Zhang, S. Zhou, Q. Liu, X. Li, L. Cai, G. Liang, Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose-induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. Diabetes 63(10), 3497–3511 (2014). https://doi.org/10.2337/db13-1577
H. Nie, C. Song, D. Wang, S. Cui, T. Ren, Z. Cao, Q. Liu, Z. Chen, X. Chen, Y. Zhou, MicroRNA-194 inhibition improves dietary-induced non-alcoholic fatty liver disease in mice through targeting on FXR. Biochim. Biophys. Acta 1863(12), 3087–3094 (2017). https://doi.org/10.1016/j.bbadis.2017.09.020
P.J. Hunt, A.M. Richards, M.G. Nicholls, T.G. Yandle, R.N. Doughty, E.A. Espiner, Immunoreactive amino-terminal pro-brain natriuretic peptide (NT-PROBNP): a new marker of cardiac impairment. Clin. Endocrinol. 47(3), 287–296 (1997)
H. Valadi, K. Ekstrom, A. Bossios, M. Sjostrand, J.J. Lee, J.O. Lotvall, Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9(6), 654–659 (2007). https://doi.org/10.1038/ncb1596
M. Whitham, B.L. Parker, M. Friedrichsen, J.R. Hingst, M. Hjorth, W.E. Hughes, C.L. Egan, L. Cron, K.I. Watt, R.P. Kuchel, N. Jayasooriah, E. Estevez, T. Petzold, C.M. Suter, P. Gregorevic, B. Kiens, E.A. Richter, D.E. James, J.F.P. Wojtaszewski, M.A. Febbraio, Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell. Metab. 27(1), 237–251 e234 (2018). https://doi.org/10.1016/j.cmet.2017.12.001
K.M. Davies, M. Strauss, B. Daum, J.H. Kief, H.D. Osiewacz, A. Rycovska, V. Zickermann, W. Kuhlbrandt, Macromolecular organization of ATP synthase and complex I in whole mitochondria. Proc. Natl. Acad. Sci. USA 108(34), 14121–14126 (2011). https://doi.org/10.1073/pnas.1103621108
F.C. Yin, H.A. Spurgeon, K. Rakusan, M.L. Weisfeldt, E.G. Lakatta, Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am. J. Physiol. 243(6), H941–H947 (1982). https://doi.org/10.1152/ajpheart.1982.243.6.H941
F.S. Loffredo, M.L. Steinhauser, S.M. Jay, J. Gannon, J.R. Pancoast, P. Yalamanchi, M. Sinha, C. Dall’Osso, D. Khong, J.L. Shadrach, C.M. Miller, B.S. Singer, A. Stewart, N. Psychogios, R.E. Gerszten, A.J. Hartigan, M.J. Kim, T. Serwold, A.J. Wagers, R.T. Lee, Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153(4), 828–839 (2013). https://doi.org/10.1016/j.cell.2013.04.015
S. De Rosa, B. Arcidiacono, E. Chiefari, A. Brunetti, C. Indolfi, D.P. Foti, Type 2 diabetes mellitus and cardiovascular disease: genetic and epigenetic links. Front. Endocrinol. 9, 2 (2018). https://doi.org/10.3389/fendo.2018.00002
C.L. Kurtz, B.C. Peck, E.E. Fannin, C. Beysen, J. Miao, S.R. Landstreet, S. Ding, V. Turaga, P.K. Lund, S. Turner, S.B. Biddinger, K.C. Vickers, P. Sethupathy, MicroRNA-29 fine-tunes the expression of key FOXA2-activated lipid metabolism genes and is dysregulated in animal models of insulin resistance and diabetes. Diabetes 63(9), 3141–3148 (2014). https://doi.org/10.2337/db13-1015
Z. Yang, L. Wu, X. Zhu, J. Xu, R. Jin, G. Li, F. Wu, MiR-29a modulates the angiogenic properties of human endothelial cells. Biochem. Biophys. Res. Commun. 434(1), 143–149 (2013). https://doi.org/10.1016/j.bbrc.2013.03.054
Q. Lin, Z. Yun, The hypoxia-inducible factor pathway in adipocytes: the role of HIF-2 in adipose inflammation and hypertrophic cardiomyopathy. Front. Endocrinol. 6, 39 (2015). https://doi.org/10.3389/fendo.2015.00039
V.J. Dzau, Autocrine and paracrine mechanisms in the pathophysiology of heart failure. Am. J. Cardiol. 70(10), 4C–11C (1992)
J.F. Keaney Jr., M.G. Larson, R.S. Vasan, P.W. Wilson, I. Lipinska, D. Corey, J.M. Massaro, P. Sutherland, J.A. Vita, E.J. Benjamin, S. Framingham, Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 23(3), 434–439 (2003). https://doi.org/10.1161/01.ATV.0000058402.34138.11
K. Denzer, M.J. Kleijmeer, H.F. Heijnen, W. Stoorvogel, H.J. Geuze, Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell. Sci. 113(Pt 19), 3365–3374 (2000)
X. Wang, H. Gu, W. Huang, J. Peng, Y. Li, L. Yang, D. Qin, K. Essandoh, Y. Wang, T. Peng, G.C. Fan, Hsp20-mediated activation of exosome biogenesis in cardiomyocytes improves cardiac function and angiogenesis in diabetic mice. Diabetes 65(10), 3111–3128 (2016). https://doi.org/10.2337/db15-1563
E.E. Creemers, A.J. Tijsen, Y.M. Pinto, Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 110(3), 483–495 (2012). https://doi.org/10.1161/CIRCRESAHA.111.247452
M.J. Rahman, D. Regn, R. Bashratyan, Y.D. Dai, Exosomes released by islet-derived mesenchymal stem cells trigger autoimmune responses in NOD mice. Diabetes 63(3), 1008–1020 (2014). https://doi.org/10.2337/db13-0859
G. Zhang, Q. Wang, R. Xu, Therapeutics based on microRNA: a new approach for liver cancer. Curr. Genom. 11(5), 311–325 (2010). https://doi.org/10.2174/138920210791616671
Acknowledgements
This study was supported by the match funding for the Key Laboratory of Myocardial Ischemia, Chinese Ministry Education (KL201316), The Heilongjiang Postdoctoral Fund (LBH-Z16133), and Chinese Postdoctoral Science Foundation (2017M611395).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Procedures involving the clinical trial and animal experiments were approved by Human Ethics Committee and Animal Policy and Welfare of Harbin Medical University Committee.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
These authors contributed equally: Fengqin Li, Kuikui Zhang.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, F., Zhang, K., Xu, T. et al. Exosomal microRNA-29a mediates cardiac dysfunction and mitochondrial inactivity in obesity-related cardiomyopathy. Endocrine 63, 480–488 (2019). https://doi.org/10.1007/s12020-018-1753-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1753-7