Abstract
Purpose
Distant metastases (DM) from DTC occur in 5–25% of cases and are correlated to lower survival; the prognostic significance of the temporal onset of DM is unclear. Our aim was to retrospectively analyze the prevalence of DM and to assess the prognostic role of the timing of manifestation of DM regarding the outcome.
Materials and methods
We included 174 patients (mean age 64 years). According to the time of manifestation, DM were divided in two groups: synchronous DM (SDM, n = 108) defined as metastases present at initial diagnosis and metachronous DM (MDM, n = 66) as diagnosed during follow-up. SDM were further sub grouped in pre-RAIT when diagnosed during pre-surgery work-up (n = 35) and baseline-RAIT when detected by first whole body scan after RAIT (n = 73). Disease-specific survival (DSS) was analyzed using the Kaplan–Meier method.
Results
Total RAI activities and number of treatments were significantly higher in MDM, also loss of RAI avidity was more frequent in MDM. Forty-four patients died during follow-up, of which 41 were DTC-related deaths, 5-year and 10-year DSS were 80% and 56%. On univariate analysis MDM had significantly shorter DSS; also histotype and RAI avidity were significant risk factors of impaired survival. On multivariate analysis, only loss of RAI avidity remained as independent negative predictor (p = 0.043). Considering SDM, DSS was significantly shorter in pre-RAIT group than baseline-RAIT (p = 0.004). Instead there was no significant difference between pre-RAIT-SDM and MDM in survival outcome (p = 0.875).
Conclusions
In DTC with DM, loss of RAI uptake has an important role in survival. No significant difference in survival outcome was discovered between SDM and MDM; but, among SDM, pre-RAIT had significant shorter DSS than baseline-RAIT.
Similar content being viewed by others
Introduction
Differentiated thyroid cancer (DTC) is the most common endocrine malignancy accounting for 1% of all cancers diagnosed each year [1] and is considered a slowly growing disease with favorable prognosis. Standard treatment for DTC consists of total thyroidectomy with or without lymphadenectomy followed by radioiodine therapy (RAIT) of remnant tissue when indicated [2]. The overall prognosis of DTC is excellent, with a 5-year survival rates for DTC of 99.8% for localized tumors, 97% for tumors with regional metastases, and 57.3% for tumors with distant metastases [3]. Metastatic DTC patients are a group of patients occurring in 5–25% of patients with lower survival rate and they require an early and appropriate treatment to improve their prognosis [4,5,6,7].
Treatment of DTC patients presenting with distant metastases (DM) is usually individualized according to DM features and disease progression; RAIT may be an effective therapy for metastatic DTC if metastatic lesions are RAI-avid and may contribute significantly to their life expectancy [8]. Other therapeutic modalities for metastatic lesions include surgical resection, external radiation therapy, and selective embolization. Lungs are the most common site of distant metastases followed by bone; other rare localizations include brain, liver, and kidney.
The prognostic significance of the temporal onset of distant metastases in DTC is yet unclear due to the controversial results present in literature [9,10,11,12,13,14]. Depending on the time of their onset, metastases are divided in synchronous and metachronous: synchronous distant metastases (SDM) include metastases detected at the time of initial thyroid cancer diagnosis and represent 1–3% of all patients, while metachronous distant metastases (MDM) are recognized after the initial treatment and occur in 7–23% of patients [6, 7].
Our aim was to retrospectively analyze in a consecutive cohort of patients evaluated in our institute, the prevalence of metastatic disease in DTC patients and to assess the prognostic role of the timing of manifestation of distant metastases regarding the outcome of these patients.
Materials and methods
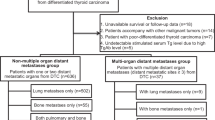
We retrospectively screened 3600 patients who underwent RAIT for DTC after total or nearly total thyroidectomy from January 1997 until January 2017 using our institutional Radiology Information System (RIS). They were admitted to the Nuclear Medicine Department of our Institution for the ablation of thyroid remnant according to EAMN (European Association of Nuclear Medicine) guidelines [15]. Among these patients, we found 174 patients with distant metastases of DTC (110 female; 64 male; sex ratio F: M 1.7:1). Average age of patients at diagnosis of thyroid carcinoma was 58 ± 16 with a range from 11 to 85 years; only 3 patients were pediatric. All distant metastases were confirmed by histology when available and/or other imaging modalities (computed tomography, positron emission tomography, bone scintigraphy, magnetic resonance imaging). All patients underwent total thyroidectomy and had histopathological diagnosis of DTC: 74 classic variant of papillary carcinoma, 39 follicular variant of papillary carcinoma, 40 follicular carcinoma, 15 aggressive papillary variants (6 tall cells variant of papillary carcinoma, 3 sclerosing diffuse variant of papillary carcinoma and 6 poorly differentiated carcinoma), and 6 Hurtle cell carcinoma. Seventy-two patients underwent also lymphadenectomy, central in 29 cases and lateral in 43, showing nodal disease in 52 cases. Tumor size was 21 ± 22 mm (range 1.5–98 mm) and multicentricity of lesions was present in 63 cases (36%). In patients without antithyroglobulin antibody (AbTg) interference, serum thyroglobulin (Tg) at the time of first therapy was 350 ± 329 ng/mL (range 0.1–1001); AbTg were positive in 33 patients (19%). The administered activity of RAI first ablation treatments ranged from 0.9 to 3.7 GBq (average 3.3 GBq) and it was established according to the risk class based on the TNM staging of the American Joint Committee on Cancer/International Union against Cancer currently in use [16]. One hundred and fifty-eight patients underwent levothyroxine withdrawal for 40 days, replaced by levo-triiodothyronine in the first 20 days, while in sixteen patients recombinant human thyrotropin (rhTSH-Thyrogen, Genzyme Corporation) was administered intramuscularly with a dose of 0.9 mg over 2 consecutive days during treatment with levothyroxine; RAI was administered the day after the second injection. All patients followed a low-iodine diet for 2 weeks and average serum thyrotropin (TSH) concentration was 61.6 UI/L before first radioiodine administration. The average number of RAIT per patients was 4.7 ± 2.3 (range 1–13) and the average cumulative RAI activity administered per patient was 32.6 ± 41 GBq (range 1.1–114 GBq). Baseline features of all patients are described in Table 1. The average follow-up time was 120 ± 69 months (range 12–242 months, median 72 months). Loss of RAI avidity was defined as the absence of uptake at whole body scan after RAIT in presence of disease demonstrated with other conventional imaging or tumor marker (Tg); this phenomenon was considered present at any time of presentation during all the course of the follow-up.
Patients were retrospectively divided into two groups based on the temporal onset of metastatic disease: SDM were defined as the metastases present at initial diagnosis (before thyroid surgery or after radiometabolic therapy at first whole body scan), and MDM as metastases diagnosed by subsequent whole body scans or other radiologic examinations after initial diagnosis and RAIT remnant ablation during follow-up period.
SDM patients were further divided in two subgroups: patients with metastases diagnosed during pre-surgery work-up by radiologic examinations or due to symptomatic appearance, (pre-RAIT group), and patients with metastases detected by whole body scan during initial RAI remnant ablation, (baseline-RAIT group).
Statistical analysis
The numeric variables were described as mean, standard deviation, minimum, and maximum. The descriptive analysis of categorical variables comprised the calculation of simple and relative frequencies.
The statistical significance of the continuous variables was tested with a Student’s t-test or Mann–Whitney’s U-test and a Chi-square or Fisher’s exact test was performed for the categorical variables. A P-value of ≤ 0.05 was considered as statistically significant.
Survival analysis was performed with the Kaplan–Meier method and compared by the log rank test with a significance level set at p < 0.05. Disease-specific survival (DSS) was assessed from the onset of distant metastases, and death from DTC was considered as an event. Multivariate analysis using with the Cox proportional hazards model was performed for significant factors in univariate analysis. The hazard ratio, 95% confidence interval (CI), and P-value were reported. Nodal disease was not considered in this statistical analysis due to the shared notion that this feature is not relevant for prognostic in comparison with distant metastases. Statistical analysis was carried out using Statistical Package for Social Science (SPSS) version 23.0 for Windows (IBM, Chicago, Illinois, USA).
Results
Considering all 3600 patients evaluated, 174 (4.8%) showed distant metastases of DTC: 61 in the bone, 86 in the lung, 17 in bone and lung simultaneously and 10 in other sites. Other localizations were as follows: 1 kidney; 1 muscle tissue; 1 brain; 1 bone and brain; 1 bone and liver; 1 muscle and bone; 2 bone, lung and brain; and 2 brain and lung. Histological confirmation of metastases was achieved in 72 patients, while in another 102 cases other imaging modalities (whole body scintigraphy, CT, MRI, PET) confirmed the presence of the metastases. One hundred and eight patients (62%) had SDM, while 66 patients (38%) developed MDM after first radiometabolic therapy during the follow-up. The mean time interval between initial diagnosis and onset of metastatic disease in patients with MDM was 4.8 ± 3.8 years (range 1–18). The SDM and MDM groups were well balanced regarding the features of sex, tumor size, multicentricity, type of surgery (central and/or lateral lymphadenectomy), lymph nodal involvement, histological type of DTC, site of metastatic disease, presence of AbTg, and first RAI activities injected (Table 2). Classic variant of papillary carcinoma was the most common histotype in both groups with 39 cases in SDM group and 35 in MDM; the other most frequent subtypes were follicular and follicular variant of papillary carcinoma. Considering the SDM, metastases were localized in the bone in 37 patients, in the lung in 54, in the bone and lung together in 12 and in other sites in 5 cases; instead in MDM, metastases were localized in the bone in 24 patients, in the lung in 32, in the bone and lung together in 5, and in other sites in 5 cases. Female population was significantly more frequent in SDM group in comparison with MDM; total RAI activities administrated and total number of radiometabolic treatments were significantly higher in MDM than SDM (p = 0.013 and p = 0.014, respectively), while stimulated Tg level was significantly higher in SDM (p < 0.001). In addition, loss of RAI avidity during follow-up was more frequent in MDM patients (50% of cases) in comparison with SDM (16% of cases) (p < 0.001). During follow-up, patients underwent several treatment aside from radioiodine: among SDM 12 underwent local therapy (as external radiotherapy or surgery) and 4 systemic therapy (as chemotherapy and/or tyrosine-kinase inhibitors); while among MDM, 10 underwent local therapy and 3 systemic therapy.
Survival outcomes
After an average time of 10 years, 44 patients died during follow-up, of which 41 were DTC-related deaths. The median DSS from the diagnosis of distant metastases was 8 years (95%CI 2-16.6); 5-year and 10-year DSS were 80% and 56%. On univariate analysis, patients with MDM had significantly shorter survival time from the onset of distant metastases compared to patients with SDM (median DSS 5 vs. 7 years, p = 0.039); 5-year and 10-year DSS rates were 83% and 61% for patients with SDM, and 76% and 51% for patients with MDM, respectively (Fig. 1). Histological subtype of DTC and loss of RAI avidity were the other only significant risk factors of impaired survival in our analysis: follicular carcinoma had shorter tumor related survival than the patients with papillary carcinoma (median DSS 4 and 9 years, respectively, p = 0.001) and the same evidence was obtained comparing lost RAI avidity group and RAI avid group (median DSS 6 and 6.5 years, respectively, p = 0.011, Fig. 2a). Age, gender, tumor size, multifocality, and site of metastatic disease (considering the two most frequent localizations as bone and lung), had no impact on disease-related outcome (Table 3). Also treatment during follow-up did not influence survival outcome. On multivariate analysis, only loss of RAI avidity remained as independent negative predictors for tumor related survival (p = 0.043). In both SDM and MDM groups, loss of RAI was found to be a prognostic factor associated with a worst DSS (Fig. 2b, e 2c).
Considering SDM group, 35/108 (32%) metastases were identified before thyroid surgery as first step which led to the diagnosis of DTC (pre-RAIT group), while the other 73/108 (68%) were detected in the first whole body scan after treatment (baseline-RAIT group). Among 35 pre-RAIT DM patients, 17 were asymptomatic and metastases were incidentally detected with other imaging techniques performed for other reasons not related to thyroid diseases and 18 were diagnosed due to the appearance of signs/symptoms. These symptoms/signs included bone pain (n = 7), dyspnea (n = 4), cough (n = 2), bone fractures (n = 4), and skin nodules (n = 1). In the pre-RAIT group, there were significantly more patients with follicular carcinoma and bone metastases in comparison with baseline-RAIT group and initial stimulated Tg value was significantly higher (800 ng/mL vs. 344 ng/mL). In baseline-RAIT patients, lung was the most frequent site of DM (60% of cases) and papillary the most frequent histotype (45%). Also RAI avidity was significantly different between these two groups: loss of RAI avidity was more common in pre-RAIT SDM (9 of 35 patients in comparison with only 8 of 108 in baseline-RAIT group). There were no significant differences in sex, age, multicentricity of cancer, initial surgery (central, lateral lymphadenectomy), tumor size, first dose of RAI administrated, total RAI administrated, total number of therapies, and number of patients with positive AbTg. (Table 4).
Survival rates were significantly different between pre-RAIT and baseline-RAIT SDM patients: DSS was significantly shorter in pre-RAIT group than baseline-RAIT with a median DSS of 9 years in first group and 4 in the other (p = 0.004, HR 0.54, 95%CI 0.27–0.98). Instead there was no significant difference between pre-RAIT and MDM in survival outcome (p = 0.875) (Fig. 3).
Discussion
Previous studies have identified several prognostic factors significantly associated with poorer survival outcome in patients with DTC, including age >45 years, follicular thyroid carcinoma, Hurthle cell variant, poor differentiation, iodine avidity, incomplete local control, and extrapulmonary DM; but some of these studies grouped and analyzed patients with DM without a division considering time of onset of DM [17,18,19,20,21]. Complete loss of radioiodine uptake capacity may indicate tumor progression as a result of transformation of the tumor to a poorly differentiated state with consequently mismatch between 18F-FDG PET/CT and radioiodine imaging (“flipflop phenomenon) [22, 23].
In our 20-year experience considering all DTC patients treated in our department, we found 4.8% of patients with distant metastases from DTC, in concordance with other studies [6, 7]: among them, more than half (62%) showed synchronous metastases. Presence of distant metastases is a well demonstrated factor worsening the prognosis in patients with DTC [24, 25] but there are few data regarding the prognostic relevance of onset time and these data are controversial. Initial studies [5, 9, 10, 26] have demonstrated no significant difference in survival rates between synchronous and metachronous metastases. In contrast with this, subsequent studies [12,13,14, 17, 27] observed a more favorable outcome in patients presenting with initial distant metastatic DTC compared to patients who developed metachronous metastases during follow-up. One of the limitations of these studies, which reduces the possibility of a direct comparison, is the different threshold used to categorize the distant metastases in SDM and MDM: some authors used a cut-off of 6 months before or after thyroid cancer diagnosis [5, 9, 10], other of 12 months [11, 14]. Moreover, another limitation is that some articles are specifically focused on one site of metastases (bone or lung) only excluding all others localizations [12, 13, 27]. In our study, we decided to include all sites of distant metastases and we arbitrarily divided our patients in two groups: SDM and MDM, using as reference cut-off the first whole body scan after RAI, in concordance with other studies [26, 27]. Despite the evidence of a better survival outcome (DSS) in SDM patients than MDM, this difference was not significantly different in multivariate analysis, similarly to other published results [11, 12]. A possible explanation of a different outcome between SDM and MDM groups is the heterogeneous composition of SDM group, which include patients with DM diagnosed before RAIT and at first RAI whole body scintigraphy. The only independent prognostic factor influencing DSS was RAI avidity: in patients with lost RAI avidity during the course of disease, the prognosis is significantly worse and these results are confirmed both for SDM and MDM categories (Fig. 2). Loss of RAI avidity happens when dedifferentiation occurs in thyroid cancer [28] and its prognostic role is well established and seems to have a fundamental function also in metastatic DTC [2, 17, 18].
To make a more complete evaluation, we further subdivided SDM patients in two subgroups: pre-RAIT and baseline-RAIT groups founding different features regarding stimulated Tg value at staging, site of metastases, histotype, and RAI avidity. Pre-RAIT SDM patients had more frequently high Tg, bone metastases, follicular carcinoma, and lost RAI avidity; all factors, support the idea of an aggressive behavior of pre-RAIT DM.
Also Choi et al. [27] evaluated metastatic patients dividing distant metastases in pre-radioiodine and post-radioiodine therapy (pre-RAIT and post-RAIT) groups depending on the detection of metastases: pre-RAIT metastases were clinically detected before an initial RAIT, while post-RAIT was detected on post ablation whole body scan or during follow-up period. They demonstrated that pre-RAIT groups had significantly poorer prognosis. Also in our analysis, we showed that distant metastases detected before surgery and RAIT had worse survival outcome compared to metastases detected at first whole body scan. This may be the reason why synchronous/metachronous categories have been reported as inconclusive prognostic factors in several articles in literature, because they included in the same group metastases recognized before RAI and at the first therapeutic whole body scan.
Moreover, patients who have no symptoms or skeletal related events and with a diagnosis of skeletal metastases detected by initial RAIT demonstrate excellent responses and much better survival than patients who demonstrate bone metastasis before the first RAIT [12, 29, 30]. Patients who demonstrate bone metastasis at the initial whole body RAI scintigraphy often include lesions with no structural correlation at radiological imaging, which is known to have no prognostic significance [31]; another point is that those metastases discovered by whole body scans are often considered more “RAI naive” and so they are generally more RAI responsive and have higher RAI avidity [7, 17]. These evidences were confirmed in our analysis where we found that only 8/73 (11%) baseline-RAIT SDM patients lost RAI avidity during the course of disease in contrast with 9/35 (26%) in pre-RAIT SDM.
Probably the division of metastatic DTC patients in three groups (pre-RAIT, baseline-RAIT SDM, and MDM) may be the best choice considering different metabolic and histological features of DTC. Our paper evaluated a larger cohort than in previous reports and included patients with DM at different sites. Cho et al. [27] firstly divided lung DM patients in pre-operative, immediate, and late metastases, demonstrating a worse prognosis in the first group despite only 11 cases. Lang et al. [18] compared pre-operative metastases and metastases discovered at whole body scan showing poorer prognosis in univariate analysis in pre-operative group, but this evidence was not confirmed in multivariate analysis. Our findings suggest that the clinical characteristics of SDM patients in the two groups (pre-RAIT and baseline-RAIT) are quite different with more aggressive characteristics in pre-RAIT, in presence of higher stimulated Tg, bone localization, follicular histotype, and loss of RAI avidity.
In addition, the timing of detection of distant metastases is a clinical feature with a valuable prognostic role. SDM detected at first whole body scan had better survival rates than SDM detected before RAIT, while no significant differences were recognized between SDM and MDM categories.
This study had several limitations, including the retrospective nature of the study design and the use of heterogeneous management approaches over a relatively long period.
In conclusion, RAI avid DM responded much better than not RAI avid DM and this evidence is present true in both SDM and MDM. Timing of onset of distant metastases may add an important prognostic feature to risk stratification in DTC. No significant difference in survival outcome was discovered between SDM and MDM; but, among SDM patients, pre-RAIT DM had significant shorter DSS than baseline-RAIT.
References
S.I. Sherman, Thyroid carcinoma. Lancet 361, 501–511 (2003)
B.R. Haugen, E.K. Alexander, K.C. Bible, G.M. Doherty, S.J. Mandel, Y.E. Nikiforov et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 26, 1–133 (2016)
E.P. Simard, E.M. Ward, R. Siegel, A. Jemal, Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J. Clin. 62, 118–128 (2012)
E. Sampson, J.D. Brierley, L.W. Le, L. Rotstein, R.W. Tsang, Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer 110, 1451–1456 (2007)
M. Schlumberger, M. Tubiana, F. De Vathaire, C. Hill, P. Gardet, J.P. Travagli et al. Long term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 63, 960–967 (1986)
D. Albano, F. Bertagna, M. Bertoli, M. Bonacina, R. Durmo, E. Cerudelli et al. Possible delayed diagnosis and treatment of metastatic differentiated thyroid cancer by adopting the 2015 ATA guidelines. Eur. J. Endo. (2018) https://doi.org/10.1530/EJE-18-0253
M. Haq, C. Harmer, Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin. Endocrinol. 63, 87–93 (2005)
F.A. Verburg, M. Luster, Radioactive iodine (RAI) therapy for metastatic differentiated thyroid cancer. Best. Pract. Res Clin. Endocrinol. Metab. 31, 279–290 (2017)
M. Shoup, A. Stojadinovic, A. Nissan, R.A. Ghossein, S. Freedman, M.F. Brennan et al. Prognostic indicators of outcomes in patients with distantmetastasesfromdifferentiated thyroid carcinoma. J. Am. Coll. Surg. 197, 191–197 (2003)
A. Stojadinovic, M. Shoup, R.A. Ghossein, A. Nissan, M.F. Brennan, J.P. Shah et al. The role of operations for distantly metastatic well-differentiated thyroid carcinoma. Surgery 131, 636–643 (2002)
J.M. Mihailovic, L.J. Stefanovic, M.D. Malesevic, M.D. Erak, D.D. Tesanovic, Metastatic differentiated thyroid carcinoma: clinical management and outcome of disease in patients with initial and late distant metastases. Nucl. Med. Commun. 30, 558–564 (2009)
Y. Orita, I. Sugitani, M. Matsuura, M. Ushijima, K. Tsukahara, Y. Fujimoto et al. Prognostic factors and the therapeutic strategy for patients with bone metastasis from differentiated thyroid carcinoma. Surgery 147, 424–431 (2010)
F. Pitoia, F. Bueno, G. Cross, Long-term survival and low-effective cumulative radioiodine doses to achieve remission in patients with 131iodine-avid lung metastasis from differentiated thyroid cancer. Clin. Nucl. Med. 39, 784–790 (2014)
A. Sabet, I. Binse, S. Dogan, A. Koch, S.J. Rosenbaum-Krumme, H.J. Biersack et al. Distinguishing sinchronous from metachronous manifestation of distant metastases: a prognostic feature in differentiated thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 44, 190–195 (2017)
M. Luster, S.E. Clarke, M. Dietlein, M. Lassmann, P. Lind, W.J. Oyen et al. European Association of Nuclear Medicine (EANM). Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 35, 1941–1959 (2008)
S.B. Edge, D.R. Byrd, C.C. Compton, A. G. Fritz, F. L. Greene, A. Trotti (eds.) AJCC Cancer Staging Manual. 7th edn. (Springer, New York, 2010).
J. Lee, E.Y. Soh, Differentiated thyroid carcinoma presenting with distant metastasis at initial diagnosis clinical outcomes and prognostic factors. Ann. Surg. 251, 114–119 (2010)
B.H. Lang, K.P. Wong, C.Y. Cheung, K.Y. Wan, C.Y. Lo, Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann. Surg. Oncol. 20, 1329–1335 (2013)
I.J. Nixon, M.M. Whitcher, F.L. Palmer, R.M. Tuttle, A.R. Shaha, J.P. Shah et al. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid 22, 884–889 (2012)
S.F. Dinneen, M.J. Valimaki, E.J. Bergstralh, J.R. Goellner, C.A. Gorman, I.D. Hay, Distant metastases in papillary thyroidcarcinoma:100 cases observed at one institution during 5 decades. J. Clin. Endocrinol. Metab. 80, 2041–2045 (1995)
C. Durante, N. Haddy, E. Baudin, S. Leboulleux, D. Hartl, J.P. Travagli et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 91, 2892–2899 (2006)
U. Feine, R. Lietzenmayer, J.P. Hanke, H. Wöhrle, W. Müller-Schauenburg, 18FDG whole body PET in differentiated thyroid carcinoma. Flipflop in uptake patterns of 18FDG and 131I. Nuklearmedizin 34, 127–134 (1995)
F. Bertagna, D. Albano, G. Bosio, A. Piccardo, B. Dib, R. Giubbini, 18F-FDG-PET/CT in patients affected by differentiated thyroid carcinoma with positive thyroglobulin level and negative 131I whole body scan. it’s value confirmed by a bicentric experience. Curr. Radiopharm. 9, 228–234 (2016)
C.I. Lundgren, P. Hall, P.W. Dickman, J. Zedenius, Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case–control study. Cancer 106, 524–531 (2006)
I.D. Hay, E.J. Bergstralh, J.R. Goellner, J.R. Ebersold, C.S. Grant, Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114, 1050–1057 (1993)
M.Y. Do, Y. Rhee, D.J. Kim, C.S. Kim, K.H. Nam, C.W. Ahn et al. Clinical features of bone metastases resulting from thyroid cancer: a review of 28 patients over a 20-year period. Endocr. J. 52, 701–707 (2005)
S.W. Cho, H.S. Choi, G.J. Yeom, J.A. Lim, J.H. Moon, D.J. Park et al. Long-term prognosis of differentiated thyroid cancer with lung metastasis in Korea and its prognostic factors. Thyroid 24, 277–286 (2014)
G. Riesco-Eizaguirre, P. Gutierrez-Martinez, M.A. Garcia-Cabezas, M. Nistal, P. Santisteban, The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na + /I- targeting to the membrane. Endocr. Relat. Cancer 13, 257–269 (2006)
Y.M. Choi, W.G. Kim, H. Kwoon, M.J. Jeon, J.J. Lee, J.S. Ryu et al. Early prognostic factors at the time of diagnosis of bone metastasis in patients with bone metastases of differentiated thyroid carcinoma. Eur. J. End. 175, 165–172 (2016)
E. Hindie, P. Zanotti-Fregonara, I. Keller, F. Duron, J.Y. Devaux, M. Calzada-Nocaudie et al. Bone metastases of differentiated thyroid cancer: impact of early 131I-based detection on outcome. Endocr. Relat. Cancer 14, 799–807 (2007)
E. Robenshtok, A. Farooki, R.K. Grewal, R.M. Tuttle, Natural history of small radioiodine-avid bone metastases that have no structural correlate on imaging studies. Endocrine 47, 266–272 (2014)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participant included in the study
Rights and permissions
About this article
Cite this article
Albano, D., Panarotto, M.B., Durmo, R. et al. Clinical and prognostic role of detection timing of distant metastases in patients with differentiated thyroid cancer. Endocrine 63, 79–86 (2019). https://doi.org/10.1007/s12020-018-1713-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1713-2