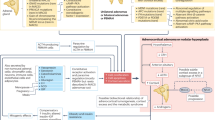
Abstract
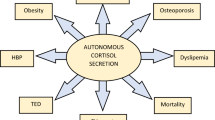
Adrenal incidentalomas constitute a common clinical problem with an overall prevalence of around 2–3%, but are more common with advancing age being present in 10% of those aged 70 years. The majority of these lesions are benign adrenocortical adenomas (80%), characterized in 10–40% of the cases by autonomous cortisol hypersecretion, and in 1–10% by aldosterone hypersecretion. Several observational studies have shown that autonomous cortisol and aldosterone hypersecretion are more prevalent than expected in patients with osteopenia and osteoporosis: these patients have accelerated bone loss and an increased incidence of vertebral fractures. In contrast to glucocorticoid action, the effects of aldosterone on bone are less well understood. Recent data, demonstrating a concomitant co-secretion of glucocorticoid metabolites in patients with primary aldosteronism, could explain some of the metabolic abnormalities seen in patients with aldosterone hypersecretion. In clinical practice, patients with unexplained osteoporosis, particularly when associated with other features such as impaired glucose tolerance or hypertension, should be investigated for the possible presence of autonomous cortisol or aldosterone secretion due to an adrenal adenoma. Randomized intervention studies are needed, however, to investigate the optimum interventions for osteoporosis and other co-morbidities in these patients.


Similar content being viewed by others
Change history
10 September 2018
The original version of this article unfortunately contained a mistake in Figure 1. There is a typo in the word “osteoclastogenesis” and the word “activity” is missing in the same entity. It should be “osteoclastogenesis” instead of “osteoclestogenesis”.
References
M. Fassnacht, W. Arlt, I. Bancos, H. Dralle, J. Newell-Price, A. Sahdev, A. Tabarin, M. Terzolo, S. Tsagarakis, O.M. Dekkers, Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 175(2), G1–G34 (2016). https://doi.org/10.1530/EJE-16-0467
S.A. Paschou, A. Vryonidou, D.G. Goulis, Adrenal incidentalomas: a guide to assessment, treatment and follow-up. Maturitas 92, 79–85 (2016). https://doi.org/10.1016/j.maturitas.2016.07.017
L. Barzon, N. Sonino, F. Fallo, G. Palu, M. Boscaro, Prevalence and natural history of adrenal incidentalomas. Eur. J. Endocrinol. 149(4), 273–285 (2003)
S. Bovio, A. Cataldi, G. Reimondo, P. Sperone, S. Novello, A. Berruti, P. Borasio, C. Fava, L. Dogliotti, G.V. Scagliotti, A. Angeli, M. Terzolo, Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J. Endocrinol. Invest. 29(4), 298–302 (2006). https://doi.org/10.1007/BF03344099
V. Nuzzo, T. Attardo, G. Augello, D. Brancato, S. Camerlingo, C. Canale, F. Coretti, A. Franco, F. Giacometti, M. Gambacorta, M. Loreno, A. Maffettone, V. Provenzano, A. Zuccoli, A clinical Audit: diagnostic and epidemiological evaluation of the adrenal incidentaloma (AI). Minerva Endocrinol. (2018). https://doi.org/10.23736/S0391-1977.18.02780-3
J. Crona, F. Beuschlein, K. Pacak, B. Skogseid, Advances in adrenal tumors 2018. Endocr. Relat. Cancer 25(7), R405–R420 (2018). https://doi.org/10.1530/ERC-18-0138
F. Mantero, M. Terzolo, G. Arnaldi, G. Osella, A.M. Masini, A. Ali, M. Giovagnetti, G. Opocher, A. Angeli, A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J. Clin. Endocrinol. Metab. 85(2), 637–644 (2000). https://doi.org/10.1210/jcem.85.2.6372
E. Vassilatou, A. Vryonidou, S. Michalopoulou, J. Manolis, J. Caratzas, C. Phenekos, I. Tzavara, Hormonal activity of adrenal incidentalomas: results from a long-term follow-up study. Clin. Endocrinol. (Oxf.). 70(5), 674–679 (2009). https://doi.org/10.1111/j.1365-2265.2008.03492.x
M. Terzolo, S. Bovio, G. Reimondo, A. Pia, G. Osella, G. Borretta, A. Angeli, Subclinical Cushing’s syndrome in adrenal incidentalomas. Endocrinol. Metab. Clin. North. Am. 34(2), 423–439 (2005). https://doi.org/10.1016/j.ecl.2005.01.008.
G. Zavatta, G. Di Dalmazi, Recent Advances on subclinical hypercortisolism. Endocrinol. Metab. Clin. North Am. 47(2), 375–383 (2018). https://doi.org/10.1016/j.ecl.2018.01.003
S.H. Ahn, J.H. Kim, S.H. Baek, H. Kim, Y.Y. Cho, S. Suh, B.J. Kim, S. Hong, J.M. Koh, S.H. Lee, K.H. Song, Characteristics of adrenal incidentalomas in a large, prospective computed tomography-based multicenter study: The COAR Study in Korea. Yonsei Med. J. 59(4), 501–510 (2018). https://doi.org/10.3349/ymj.2018.59.4.501
J.W. Funder, R.M. Carey, F. Mantero, M.H. Murad, M. Reincke, H. Shibata, M. Stowasser, W.F. Young Jr, The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 101(5), 1889–1916 (2016). https://doi.org/10.1210/jc.2015-4061
V. Tsiavos, A. Markou, L. Papanastasiou, T. Kounadi, I.I. Androulakis, N. Voulgaris, A. Zachaki, E. Kassi, G. Kaltsas, G.P. Chrousos, G.P. Piaditis, A new highly sensitive and specific overnight combined screening and diagnostic test for primary aldosteronism. Eur. J. Endocrinol. 175(1), 21–28 (2016). https://doi.org/10.1530/EJE-16-0003
W. Arlt, K. Lang, A.J. Sitch, A.S. Dietz, Y. Rhayem, I. Bancos, A. Feuchtinger, V. Chortis, L.C. Gilligan, P. Ludwig, A. Riester, E. Asbach, B.A. Hughes, D.M. O’Neil, M. Bidlingmaier, J.W. Tomlinson, Z.K. Hassan-Smith, D.A. Rees, C. Adolf, S. Hahner, M. Quinkler, T. Dekkers, J. Deinum, M. Biehl, B.G. Keevil, C.H.L. Shackleton, J.J. Deeks, A.K. Walch, F. Beuschlein, M. Reincke, Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight 2(8) (2017). pii: 93136. https://doi.org/10.1172/jci.insight.93136
I.I. Androulakis, G.A. Kaltsas, G.E. Kollias, A.C. Markou, A.K. Gouli, D.A. Thomas, K.I. Alexandraki, C.M. Papamichael, D.J. Hadjidakis, G.P. Piaditis, Patients with apparently nonfunctioning adrenal incidentalomas may be at increased cardiovascular risk due to excessive cortisol secretion. J. Clin. Endocrinol. Metab. 99(8), 2754–2762 (2014). https://doi.org/10.1210/jc.2013-4064
National Clinical Guideline Centre (UK). London: Royal College of Physicians (UK); 2012.
G. Mazziotti, A. Angeli, J.P. Bilezikian, E. Canalis, A. Giustina, Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol. Metab. 17(4), 144–149 (2006). https://doi.org/10.1016/j.tem.2006.03.009
V. Shalhoub, D. Conlon, M. Tassinari, C. Quinn, N. Partridge, G.S. Stein, J.B. Lian. Glucocorticoids promote development of the osteoblast phenotype by selectively modulating expression of cell growth and differentiation associated genes. J. Cell. Biochem. 50(4), 425–440 (1992). https://doi.org/10.1002/jcb.240500411
H. Zhou, M.S. Cooper, M.J. Seibel, Endogenous glucocorticoids and bone. Bone Res. 1(2), 107–119 (2013). https://doi.org/10.4248/BR201302001
R.S. Hardy, H. Zhou, M.J. Seibel, M.S. Cooper, Glucocorticoids and bone: consequences of endogenous and exogenous excess and replacement therapy. Endocr. Rev. (2018). https://doi.org/10.1210/er.2018-00097
I. Chiodini, C.E. Vainicher, V. Morelli, S. Palmieri, E. Cairoli, A.S. Salcuni, M. Copetti, A. Scillitani, Mechanisms in endocrinology: endogenous subclinical hypercortisolism and bone: a clinical review. Eur. J. Endocrinol. 175(6), R265–R282 (2016). https://doi.org/10.1530/EJE-16-0289
E.R. Weikum, M.T. Knuesel, E.A. Ortlund, K.R. Yamamoto, Glucocorticoid receptor control of transcription: precision and plasticity via allostery. Nat. Rev. Mol. Cell Biol. 18(3), 159–174 (2017). https://doi.org/10.1038/nrm.2016.152
Z. Wu, N.L. Bucher, S.R. Farmer, Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol. Cell. Biol. 16(8), 4128–4136 (1996)
K. Ohnaka, M. Tanabe, H. Kawate, H. Nawata, R. Takayanagi, Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem. Biophys. Res. Commun. 329(1), 177–181 (2005). https://doi.org/10.1016/j.bbrc.2005.01.117
S. Hildebrandt, U. Baschant, S. Thiele, J. Tuckermann, L.C. Hofbauer, M. Rauner, Glucocorticoids suppress Wnt16 expression in osteoblasts in vitro and in vivo. Sci. Rep. 8(1), 8711 (2018). https://doi.org/10.1038/s41598-018-26300-z
W. Mak, X. Shao, C.R. Dunstan, M.J. Seibel, H. Zhou, Biphasic glucocorticoid-dependent regulation of Wnt expression and its inhibitors in mature osteoblastic cells. Calcif. Tissue Int. 85(6), 538–545 (2009). https://doi.org/10.1007/s00223-009-9303-1
I. Carcamo-Orive, A. Gaztelumendi, J. Delgado, N. Tejados, A. Dorronsoro, J. Fernandez-Rueda, D.J. Pennington, C. Trigueros, Regulation of human bone marrow stromal cell proliferation and differentiation capacity by glucocorticoid receptor and AP-1 crosstalk. J. Bone Miner. Res. 25(10), 2115–2125 (2010). https://doi.org/10.1002/jbmr.120
J. Compston, Glucocorticoid-induced osteoporosis: an update. Endocrine 61(1), 7–16 (2018). https://doi.org/10.1007/s12020-018-1588-2
A.Y. Sato, M. Cregor, J. Delgado-Calle, K.W. Condon, M.R. Allen, M. Peacock, L.I. Plotkin, T. Bellido, Protection from glucocorticoid-induced osteoporosis by anti-catabolic signaling in the absence of Sost/Sclerostin. J. Bone Miner. Res. 31(10), 1791–1802 (2016). https://doi.org/10.1002/jbmr.2869
W. Yao, W. Dai, L. Jiang, E.Y. Lay, Z. Zhong, R.O. Ritchie, X. Li, H. Ke, N.E. Lane, Sclerostin-antibody treatment of glucocorticoid-induced osteoporosis maintained bone mass and strength. Osteoporos. Int. 27(1), 283–294 (2016). https://doi.org/10.1007/s00198-015-3308-6
Z. Achiou, H. Toumi, J. Touvier, A. Boudenot, R. Uzbekov, M.S. Ominsky, S. Pallu, E. Lespessailles, Sclerostin antibody and interval treadmill training effects in a rodent model of glucocorticoid-induced osteopenia. Bone 81, 691–701 (2015). https://doi.org/10.1016/j.bone.2015.09.010
L.C. Hofbauer, F. Gori, B.L. Riggs, D.L. Lacey, C.R. Dunstan, T.C. Spelsberg, S. Khosla, Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology 140(10), 4382–4389 (1999). https://doi.org/10.1210/endo.140.10.7034
C. Swanson, M. Lorentzon, H.H. Conaway, U.H. Lerner, Glucocorticoid regulation of osteoclast differentiation and expression of receptor activator of nuclear factor-kappaB (NF-kappaB) ligand, osteoprotegerin, and receptor activator of NF-kappaB in mouse calvarial bones. Endocrinology 147(7), 3613–3622 (2006). https://doi.org/10.1210/en.2005-0717
M. Piemontese, J. Xiong, Y. Fujiwara, J.D. Thostenson, C.A. O’Brien, Cortical bone loss caused by glucocorticoid excess requires RANKL production by osteocytes and is associated with reduced OPG expression in mice. Am. J. Physiol. Endocrinol. Metab. 311(3), E587–E593 (2016). https://doi.org/10.1152/ajpendo.00219.2016
J.T. Warren, W. Zou, C.E. Decker, N. Rohatgi, C.A. Nelson, D.H. Fremont, S.L. Teitelbaum, Correlating RANK ligand/RANK binding kinetics with osteoclast formation and function. J. Cell. Biochem. 116(11), 2476–2483 (2015). https://doi.org/10.1002/jcb.25191
J. Rubin, D.M. Biskobing, L. Jadhav, D. Fan, M.S. Nanes, S. Perkins, X. Fan, Dexamethasone promotes expression of membrane-bound macrophage colony-stimulating factor in murine osteoblast-like cells. Endocrinology 139(3), 1006–1012 (1998). https://doi.org/10.1210/endo.139.3.5778
M.J. Seibel, M.S. Cooper, H. Zhou Glucocorticoid-induced osteoporosis: mechanisms, management, and future perspectives. Lancet Diabetes Endocrinol. 1(1), 59–70 (2013). https://doi.org/10.1016/S2213-8587(13)70045-7
V. Morelli, F. Donadio, C. Eller-Vainicher, V. Cirello, L. Olgiati, C. Savoca, E. Cairoli, A.S. Salcuni, P. Beck-Peccoz, I. Chiodini, Role of glucocorticoid receptor polymorphism in adrenal incidentalomas. Eur. J. Clin. Invest. 40(9), 803–811 (2010). https://doi.org/10.1111/j.1365-2362.2010.02330.x
M.S. Cooper, E.H. Rabbitt, P.E. Goddard, W.A. Bartlett, M. Hewison, P.M. Stewart, Osteoblastic 11beta-hydroxysteroid dehydrogenase type 1 activity increases with age and glucocorticoid exposure. J. Bone Miner. Res. 17(6), 979–986 (2002). https://doi.org/10.1359/jbmr.2002.17.6.979
M. Torlontano, I. Chiodini, M. Pileri, G. Guglielmi, M. Cammisa, S. Modoni, V. Carnevale, V. Trischitta, A. Scillitani, Altered bone mass and turnover in female patients with adrenal incidentaloma: the effect of subclinical hypercortisolism. J. Clin. Endocrinol. Metab. 84(7), 2381–2385 (1999). https://doi.org/10.1210/jcem.84.7.5856
I. Chiodini, M. Torlontano, V. Carnevale, G. Guglielmi, M. Cammisa, V. Trischitta, A. Scillitani, Bone loss rate in adrenal incidentalomas: a longitudinal study. J. Clin. Endocrinol. Metab. 86(11), 5337–5341 (2001). https://doi.org/10.1210/jcem.86.11.8022
G. Osella, M. Terzolo, G. Reimondo, A. Piovesan, A. Pia, A. Termine, P. Paccotti, A. Angeli, Serum markers of bone and collagen turnover in patients with Cushing’s syndrome and in subjects with adrenal incidentalomas. J. Clin. Endocrinol. Metab. 82(10), 3303–3307 (1997). https://doi.org/10.1210/jcem.82.10.4282
D. Hadjidakis, S. Tsagarakis, C. Roboti, M. Sfakianakis, V. Iconomidou, S.A. Raptis, N. Thalassinos, Does subclinical hypercortisolism adversely affect the bone mineral density of patients with adrenal incidentalomas? Clin. Endocrinol. (Oxf.). 58(1), 72–77 (2003)
L. Tauchmanova, R. Pivonello, M.C. De Martino, A. Rusciano, M. De Leo, C. Ruosi, C. Mainolfi, G. Lombardi, M. Salvatore, A. Colao, Effects of sex steroids on bone in women with subclinical or overt endogenous hypercortisolism. Eur. J. Endocrinol. 157(3), 359–366 (2007). https://doi.org/10.1530/EJE-07-0137
I. Chiodini, L. Tauchmanova, M. Torlontano, C. Battista, G. Guglielmi, M. Cammisa, A. Colao, V. Carnevale, R. Rossi, S. Di Lembo, V. Trischitta, A. Scillitani, Bone involvement in eugonadal male patients with adrenal incidentaloma and subclinical hypercortisolism. J. Clin. Endocrinol. Metab. 87(12), 5491–5494 (2002). https://doi.org/10.1210/jc.2002-020399
A. Sartorio, A. Conti, S. Ferrero, S. Giambona, T. Re, E. Passini, B. Ambrosi, Evaluation of markers of bone and collagen turnover in patients with active and preclinical Cushing’s syndrome and in patients with adrenal incidentaloma. Eur. J. Endocrinol. 138(2), 146–152 (1998)
R. Rossi, L. Tauchmanova, A. Luciano, M. Di Martino, C. Battista, L. Del Viscovo, V. Nuzzo, G. Lombardi, Subclinical Cushing’s syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J. Clin. Endocrinol. Metab. 85(4), 1440–1448 (2000). https://doi.org/10.1210/jcem.85.4.6515
G. Osella, G. Reimondo, P. Peretti, A. Ali, P. Paccotti, A. Angeli, M. Terzolo, The patients with incidentally discovered adrenal adenoma (incidentaloma) are not at increased risk of osteoporosis. J. Clin. Endocrinol. Metab. 86(2), 604–607 (2001). https://doi.org/10.1210/jcem.86.2.7178
I. Chiodini, G. Guglielmi, C. Battista, V. Carnevale, M. Torlontano, M. Cammisa, V. Trischitta, A. Scillitani, Spinal volumetric bone mineral density and vertebral fractures in female patients with adrenal incidentalomas: the effects of subclinical hypercortisolism and gonadal status. J. Clin. Endocrinol. Metab. 89(5), 2237–2241 (2004). https://doi.org/10.1210/jc.2003-031413
I. Chiodini, V. Morelli, B. Masserini, A.S. Salcuni, C. Eller-Vainicher, R. Viti, F. Coletti, G. Guglielmi, C. Battista, V. Carnevale, L. Iorio, P. Beck-Peccoz, M. Arosio, B. Ambrosi, A. Scillitani, Bone mineral density, prevalence of vertebral fractures, and bone quality in patients with adrenal incidentalomas with and without subclinical hypercortisolism: an Italian multicenter study. J. Clin. Endocrinol. Metab. 94(9), 3207–3214 (2009). https://doi.org/10.1210/jc.2009-0468
V. Morelli, C. Eller-Vainicher, A.S. Salcuni, F. Coletti, L. Iorio, G. Muscogiuri, S. Della Casa, M. Arosio, B. Ambrosi, P. Beck-Peccoz, I. Chiodini, Risk of new vertebral fractures in patients with adrenal incidentaloma with and without subclinical hypercortisolism: a multicenter longitudinal study. J. Bone Miner. Res. 26(8), 1816–1821 (2011). https://doi.org/10.1002/jbmr.398
C. Eller-Vainicher, V. Morelli, F.M. Ulivieri, S. Palmieri, V.V. Zhukouskaya, E. Cairoli, R. Pino, A. Naccarato, A. Scillitani, P. Beck-Peccoz, I. Chiodini, Bone quality, as measured by trabecular bone score in patients with adrenal incidentalomas with and without subclinical hypercortisolism. J. Bone Miner. Res. 27(10), 2223–2230 (2012). https://doi.org/10.1002/jbmr.1648
H. Vinolas, V. Grouthier, N. Mehsen-Cetre, A. Boisson, R. Winzenrieth, T. Schaeverbeke, C. Mesguich, L. Bordenave, A. Tabarin, Assessment of vertebral microarchitecture in overt and mild Cushing’s syndrome using trabecular bone score. Clin. Endocrinol. (Oxf) (2018). https://doi.org/10.1111/cen.13743
S.A. Paschou, E. Kandaraki, F. Dimitropoulou, D.G. Goulis, A. Vryonidou, Subclinical Cushing’s syndrome in patients with bilateral compared to unilateral adrenal incidentalomas: a systematic review and meta-analysis. Endocrine 51(2), 225–235 (2016). https://doi.org/10.1007/s12020-015-0776-6
V. Morelli, S. Palmieri, A.S. Salcuni, C. Eller-Vainicher, E. Cairoli, V. Zhukouskaya, A. Scillitani, P. Beck-Peccoz, I. Chiodini, Bilateral and unilateral adrenal incidentalomas: biochemical and clinical characteristics. Eur. J. Endocrinol. 168(2), 235–241 (2013). https://doi.org/10.1530/EJE-12-0777
A. Tabarin, Do the diagnostic criteria for subclinical hypercortisolism exist? Ann. Endocrinol. (Paris) 79(3), 146–148 (2018). https://doi.org/10.1016/j.ando.2018.03.013
V. Morelli, C. Eller-Vainicher, S. Palmieri, E. Cairoli, A.S. Salcuni, A. Scillitani, V. Carnevale, S. Corbetta, M. Arosio, S. Della Casa, G. Muscogiuri, A. Spada, I. Chiodini, Prediction of vertebral fractures in patients with monolateral adrenal incidentalomas. J. Clin. Endocrinol. Metab. 101(7), 2768–2775 (2016). https://doi.org/10.1210/jc.2016-1423
I. Chiodini, V. Morelli, A.S. Salcuni, C. Eller-Vainicher, M. Torlontano, F. Coletti, L. Iorio, A. Cuttitta, A. Ambrosio, L. Vicentini, F. Pellegrini, M. Copetti, P. Beck-Peccoz, M. Arosio, B. Ambrosi, V. Trischitta, A. Scillitani, Beneficial metabolic effects of prompt surgical treatment in patients with an adrenal incidentaloma causing biochemical hypercortisolism. J. Clin. Endocrinol. Metab. 95(6), 2736–2745 (2010). https://doi.org/10.1210/jc.2009-2387
I. Perogamvros, D.A. Vassiliadi, O. Karapanou, E. Botoula, M. Tzanela, S. Tsagarakis, Biochemical and clinical benefits of unilateral adrenalectomy in patients with subclinical hypercortisolism and bilateral adrenal incidentalomas. Eur. J. Endocrinol. 173(6), 719–725 (2015). https://doi.org/10.1530/EJE-15-0566
A.S. Salcuni, V. Morelli, C. Eller Vainicher, S. Palmieri, E. Cairoli, A. Spada, A. Scillitani, I. Chiodini, Adrenalectomy reduces the risk of vertebral fractures in patients with monolateral adrenal incidentalomas and subclinical hypercortisolism. Eur. J. Endocrinol. 174(3), 261–269 (2016). https://doi.org/10.1530/EJE-15-0977
V.S. Chhokar, Y. Sun, S.K. Bhattacharya, R.A. Ahokas, L.K. Myers, Z. Xing, R.A. Smith, I.C. Gerling, K.T. Weber, Hyperparathyroidism and the calcium paradox of aldosteronism. Circulation 111(7), 871–878 (2005). https://doi.org/10.1161/01.CIR.0000155621.10213.06
A. Vidal, Y. Sun, S.K. Bhattacharya, R.A. Ahokas, I.C. Gerling, K.T. Weber, Calcium paradox of aldosteronism and the role of the parathyroid glands. Am. J. Physiol. Heart Circ. Physiol. 290(1), H286–H294 (2006). https://doi.org/10.1152/ajpheart.00535.2005
A.S. Salcuni, S. Palmieri, V. Carnevale, V. Morelli, C. Battista, V. Guarnieri, G. Guglielmi, G. Desina, C. Eller-Vainicher, P. Beck-Peccoz, A. Scillitani, I. Chiodini, Bone involvement in aldosteronism. J. Bone Miner. Res. 27(10), 2217–2222 (2012). https://doi.org/10.1002/jbmr.1660
L. Petramala, L. Zinnamosca, A. Settevendemmie, C. Marinelli, M. Nardi, A. Concistre, F. Corpaci, G. Tonnarini, G. De Toma, C. Letizia, Bone and mineral metabolism in patients with primary aldosteronism. Int. J. Endocrinol. 2014, 836529 (2014). https://doi.org/10.1155/2014/836529
M. Notsu, M. Yamauchi, M. Yamamoto, K. Nawata, T. Sugimoto, Primary aldosteronism as a risk factor for vertebral fracture. J. Clin. Endocrinol. Metab. 102(4), 1237–1243 (2017). https://doi.org/10.1210/jc.2016-3206
V.C. Wu, C.H. Chang, C.Y. Wang, Y.H. Lin, T.W. Kao, P.C. Lin, T.S. Chu, Y.S. Chang, L. Chen, K.D. Wu, S.J. Chueh, Risk of fracture in primary aldosteronism: a population-based cohort study. J. Bone Miner. Res. 32(4), 743–752 (2017). https://doi.org/10.1002/jbmr.3033
S. Beavan, A. Horner, S. Bord, D. Ireland, J. Compston, Colocalization of glucocorticoid and mineralocorticoid receptors in human bone. J. Bone Miner. Res. 16(8), 1496–1504 (2001). https://doi.org/10.1359/jbmr.2001.16.8.1496
M.K. Agarwal, F. Mirshahi, M. Mirshahi, S. Bracq, J. Chentoufi, M. Hott, A. Jullienne, P.J. Marie, Evidence for receptor-mediated mineralocorticoid action in rat osteoblastic cells. Am. J. Physiol. 270(4 Pt 1), C1088–C1095 (1996)
C. Maniero, A. Fassina, V. Guzzardo, L. Lenzini, G. Amadori, M.R. Pelizzo, C. Gomez-Sanchez, G.P. Rossi, Primary hyperparathyroidism with concurrent primary aldosteronism. Hypertension 58(3), 341–346 (2011). https://doi.org/10.1161/HYPERTENSIONAHA.111.173948
J. Brown, I.H. de Boer, C. Robinson-Cohen, D.S. Siscovick, B. Kestenbaum, M. Allison, A. Vaidya, Aldosterone, parathyroid hormone, and the use of renin-angiotensin-aldosterone system inhibitors: the multi-ethnic study of atherosclerosis. J. Clin. Endocrinol. Metab. 100(2), 490–499 (2015). https://doi.org/10.1210/jc.2014-3949
E. Fischer, A. Hannemann, R. Rettig, W. Lieb, M. Nauck, A. Pallauf, M. Bidlingmaier, F. Beuschlein, H. Wallaschofski, M. Reincke, A high aldosterone to renin ratio is associated with high serum parathyroid hormone concentrations in the general population. J. Clin. Endocrinol. Metab. 99(3), 965–971 (2014). https://doi.org/10.1210/jc.2013-3214
P.H. Law, Y. Sun, S.K. Bhattacharya, V.S. Chhokar, K.T. Weber, Diuretics and bone loss in rats with aldosteronism. J. Am. Coll. Cardiol. 46(1), 142–146 (2005). https://doi.org/10.1016/j.jacc.2005.03.055
A.L. Runyan, V.S. Chhokar, Y. Sun, S.K. Bhattacharya, J.W. Runyan, K.T. Weber, Bone loss in rats with aldosteronism. Am. J. Med. Sci. 330(1), 1–7 (2005)
E. Rossi, C. Sani, F. Perazzoli, M.C. Casoli, A. Negro, C. Dotti, Alterations of calcium metabolism and of parathyroid function in primary aldosteronism, and their reversal by spironolactone or by surgical removal of aldosterone-producing adenomas. Am. J. Hypertens. 8(9), 884–893 (1995). https://doi.org/10.1016/0895-7061(95)00182-O
L.D. Carbone, J.D. Cross, S.H. Raza, A.J. Bush, R.J. Sepanski, S. Dhawan, B.Q. Khan, M. Gupta, K. Ahmad, R.N. Khouzam, D.A. Dishmon, J.P. Nesheiwat, M.A. Hajjar, W.A. Chishti, W. Nasser, M. Khan, C.R. Womack, T. Cho, A.R. Haskin, K.T. Weber, Fracture risk in men with congestive heart failure risk reduction with spironolactone. J. Am. Coll. Cardiol. 52(2), 135–138 (2008). https://doi.org/10.1016/j.jacc.2008.03.039
H.H. Loh, N.A. Kamaruddin, R. Zakaria, N. Sukor, Improvement of bone turnover markers and bone mineral density following treatment of primary aldosteronism. Minerva Endocrinol. 43(2), 117–125 (2016)
N. Verheyen, M.R. Grubler, A. Meinitzer, C. Trummer, V. Schwetz, K. Amrein, H.P. Dimai, W. Marz, C. Catena, D. von Lewinski, J. Voelkl, I. Alesutan, A. Fahrleitner-Pammer, H. Brussee, S. Pilz, A. Tomaschitz, Effect of eplerenone on markers of bone turnover in patients with primary hyperparathyroidism—The randomized, placebo-controlled EPATH trial. Bone 105, 212–217 (2017). https://doi.org/10.1016/j.bone.2017.08.030
J.P. Granger, S. Kassab, J. Novak, J.F. Reckelhoff, B. Tucker, M.T. Miller, Role of nitric oxide in modulating renal function and arterial pressure during chronic aldosterone excess. Am. J. Physiol. 276(1 Pt 2), R197–R202 (1999)
G. Kamalov, S.K. Bhattacharya, K.T. Weber, Congestive heart failure: where homeostasis begets dyshomeostasis. J. Cardiovasc. Pharmacol. 56(3), 320–328 (2010). https://doi.org/10.1097/FJC.0b013e3181ed064f
V.S. Chhokar, Y. Sun, S.K. Bhattacharya, R.A. Ahokas, L.K. Myers, Z. Xing, R.A. Smith, I.C. Gerling, K.T. Weber, Loss of bone minerals and strength in rats with aldosteronism. Am. J. Physiol. Heart Circ. Physiol. 287(5), H2023–H2026 (2004). https://doi.org/10.1152/ajpheart.00477.2004
A.A. Zia, G. Kamalov, K.P. Newman, J.E. McGee, S.K. Bhattacharya, R.A. Ahokas, Y. Sun, I.C. Gerling, K.T. Weber, From aldosteronism to oxidative stress: the role of excessive intracellular calcium accumulation. Hypertens. Res. 33(11), 1091–1101 (2010). https://doi.org/10.1038/hr.2010.159
A.A. Herrada, C. Campino, C.A. Amador, L.F. Michea, C.E. Fardella, A.M. Kalergis, Aldosterone as a modulator of immunity: implications in the organ damage. J. Hypertens. 29(9), 1684–1692 (2011). https://doi.org/10.1097/HJH.0b013e32834a4c75
F. Atashi, A. Modarressi, M.S. Pepper, The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem. Cells Dev. 24(10), 1150–1163 (2015). https://doi.org/10.1089/scd.2014.0484
F. Buffolo, S. Monticone, T.A. Williams, D. Rossato, J. Burrello, M. Tetti, F. Veglio, P. Mulatero. Subtype diagnosis of primary aldosteronism: is adrenal vein sampling always necessary? Int. J. Mol. Sci. 18(4) (2017). https://doi.org/10.3390/ijms18040848
L. Ceccoli, V. Ronconi, L. Giovannini, M. Marcheggiani, F. Turchi, M. Boscaro, G. Giacchetti, Bone health and aldosterone excess. Osteoporos. Int. 24(11), 2801–2807 (2013). https://doi.org/10.1007/s00198-013-2399-1
S. Pilz, K. Kienreich, C. Drechsler, E. Ritz, A. Fahrleitner-Pammer, M. Gaksch, A. Meinitzer, W. Marz, T.R. Pieber, A. Tomaschitz, Hyperparathyroidism in patients with primary aldosteronism: cross-sectional and interventional data from the GECOH study. J. Clin. Endocrinol. Metab. 97(1), E75–E79 (2012). https://doi.org/10.1210/jc.2011-2183
C. Maniero, A. Fassina, T.M. Seccia, A. Toniato, M. Iacobone, M. Plebani, R. De Caro, L.A. Calo, A.C. Pessina, G.P. Rossi, Mild hyperparathyroidism: a novel surgically correctable feature of primary aldosteronism. J. Hypertens. 30(2), 390–395 (2012). https://doi.org/10.1097/HJH.0b013e32834f0451
A.S. Salcuni, V. Carnevale, C. Battista, S. Palmieri, C. Eller-Vainicher, V. Guarnieri, F. Pugliese, G. Guglielmi, G. Desina, S. Minisola, I. Chiodini, A. Scillitani, Primary aldosteronism as a cause of secondary osteoporosis. Eur. J. Endocrinol. 177(5), 431–437 (2017). https://doi.org/10.1530/EJE-17-0417
L.A. van Mierlo, L.R. Arends, M.T. Streppel, M.P. Zeegers, F.J. Kok, D.E. Grobbee, J.M. Geleijnse, Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J. Hum. Hypertens. 20(8), 571–580 (2006). https://doi.org/10.1038/sj.jhh.1002038
F. Beuschlein, M. Reincke, W. Arlt, The impact of Connshing’s syndrome—mild cortisol excess in primary aldosteronism drives diabetes risk. J. Hypertens. 35(12), 2548 (2017). https://doi.org/10.1097/HJH.0000000000001550
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
The original version of this article was revised: The typo in Figure 1 is corrected.
Rights and permissions
About this article
Cite this article
Altieri, B., Muscogiuri, G., Paschou, S.A. et al. Adrenocortical incidentalomas and bone: from molecular insights to clinical perspectives. Endocrine 62, 506–516 (2018). https://doi.org/10.1007/s12020-018-1696-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1696-z