Abstract
Purpose
In recent years, anti-Mullerian hormone (AMH) has been considered a reliable index of ovarian reserve. There are few data on AMH values in thyroid cancer. The aim of this study was to evaluate AMH levels in pre-menopausal women with a history of low-risk thyroid cancer.
Methods
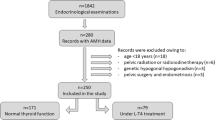
Thirty-four women (aged 40.7 ± 6.7 years) were studied 7.1 ± 0.9 years after surgery and at least one RAI treatment. A group of 23 thyroid cancer women (41.6 ± 7.4 years) who had undergone only thyroidectomy served as controls. AMH, follicle-stimulating hormone (FSH) and estradiol were assayed on days 2–3, and prolactin and progesterone levels on days 21–24 of the menstrual cycle.
Results
Pregnancy (RAI group 62%; control group 61%) and miscarriage rates (18% and 26%) were similar. AMH levels were similar in the RAI (10.7 ± 1.7 pmol/l) and control (17.5 ± 4.7 pmol/l) groups. Regular menses were reported in 41% and 52% of RAI and control subjects, respectively. Non-ovulatory cycles were noted in 26% and 35% of RAI and control women, respectively. AMH levels were found to be negatively correlated with age (RAI group P = 0.0003; control group P = 0.0001) and FSH, and positively correlated with progesterone, but not with the other hormonal parameters.
Conclusions
AMH should replace FSH in the evaluation of gonadal reserve in pre-menopausal thyroid cancer women. At present, age is the only predictor of AMH levels. About one out of two women with a history of thyroid cancer suffers from menstrual dysregulation, but infertility must be considered a low risk.


Similar content being viewed by others
References
American College Society: Cancer facts and figures 2017. American Cancer Society. Atlanta (2017)
H. Lim, S.S. Devesa, J.A. Sosa, D. Check, C.M. Kitarhara, Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA 317, 1338–1348 (2017)
B.R. Haugen, E.K. Alexander, K.C. Bible, G.M. Doherty, S.J. Mandel, Y.E. Nikiforov, F. Pacini, G.W. Randolph, A.M. Sawka, M. Schlumberger, K.G. Schuff, S.I. Sherman, J.A. Sosa, D.L. Steward, R.M. Tuttle, L. Wartofsky, 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid 26, 1–133 (2016)
R. Loevinger, T.F. Budinger, E.E. Watson, MIRD primer (Society of Nuclear Medicine, Reston 1999)
D. Casara, D. Rubello, G. Saladini, A. Piotto, M.R. Pelizzo, M.E. Girelli, B. Busnardo, Pregnancy after high therapeutic doses of iodine-131 in differentiated thyroid cancer: potential risks and recommendations. Eur. J. Nucl. Med. 30, 192–194 (1993)
M.E. Dottorini, G. Lomuscio, L. Mazzucchelli, A. Vignati, L. Colombo, Assessment of female fertility and carcinogenesis after iodine-131 therapy for differentiated thyroid carcinoma. J. Nucl. Med. 36, 21–27 (1995)
M. Schlumberger, F. De Vathaire, C. Ceccarelli, M.J. Delisle, C. Francese, J.E. Couette, A. Pinchera, C. Parmentier, Exposure to radioactive iodine-131 for scintigraphy or therapy does not preclude pregnancy in thyroid cancer patients. J. Nucl. Med. 37, 606–612 (1966)
C. Anderson, S.M. Engel, M.A. Weaver, J.P. Zevallos, H.B. Nichols, Birth rates after radioactive iodine treatment for differentiated thyroid cancer. Int. J. Cancer (2017). https://doi.org/10.1002/ijc.30917
M.B. Stone, J.B. Stanford, J.L. Lyon, J.A. VanDerslice, S.C. Alder, Childhood thyroid radioiodine exposure and subsequent infertility in the intermountain fallout cohort. Environ. Health Perspect. 121, 79–84 (2013)
J. Mihailovic, K. Nikoletic, D. Srbovan, Recurrent disease in juvenile differentiated thyroid carcinoma: prognostic factors, treatments, and outcomes. J. Nucl. Med. 55, 710–717 (2014)
C. Bal, A. Kumar, M. Tripathi, N. Chandrashekar, H. Phom, N.R. Murali, P. Chandra, G.S. Pant, High-dose radioiodine treatment for differentiated thyroid carcinoma is not associated with change in female fertility or any genetic risk to the offspring. Int. J. Radiat. Oncol. Biol. Phys. 63, 449–455 (2005)
J.M.G.-Q. Munoz, T.M. Hernandez, A.T. Cuadro, T.C. Molina, J.C. Montano, A.S. Perez, Etad de menopusia en pacientes tratadas con I131 por cancer differenciado de tiroides. Endocinol. Nutr. 57, 105–109 (2010)
J.P. Raymond, M. Izembart, V. Marliac, F. Dagousset, R.E. Merceron, M. Vulpillat, G. Vallée, Temporary ovarian failure in thyroid cancer patients after thyroid remnant ablation with radioactive iodine. J. Clin. Endocrinol. Metab. 69, 186–190 (1989)
C.T. Oliver-Williams, P.J. Steer, Racial variation in the number of spontaneous abortions before a first successful pregnancy, and effects on subsequent pregnancies. Int. J. Gynaecol. Obstet. 129, 207–212 (2015)
J.P. Garsi, M. Schlumberger, C. Rubino, M. Ricard, M. Labbé, C. Ceccarelli, C. Schvartz, M. Henri-Amar, S. Bardet, F. de Vathaire, Therapeutic administration of 131I for differentiated thyroid cancer: radiation dose to ovaries and outcome of pregnancies. J. Nucl. Med. 49, 845–852 (2008)
J.B. Trunnel, L.D. Marinelli, B.J. Duffy, R. Hill, W. Peacock, R.W. Awson, The treatment of metastatic thyroid cancer with radioactive iodine; credits and debits. J. Clin. Endocrinol. Metab. 9, 1138–1152 (1949)
C. Ceccarelli, W. Bencivelli, D. Morciano, A. Pinchera, F. Pacini, 131I therapy for differentiated thyroid cancer leads to an earlier onset of menopause: results of a retrospective study. J. Clin. Endocrinol. Metab. 86, 3512–3515 (2001)
A.M. Sawka, D.C. Lakra, J. Lea, B. Alshehri, R.W. Tsang, J.D. Brierley, S. Straus, L. Thabane, A. Gafni, S. Ezzat, S.R. George, D.P. Goldstein, A systematic review examining the effects of therapeutic radioactive iodine on ovarian function and future pregnancy in female thyroid cancer survivors. Clin. Endocrinol. 69, 479–490 (2008)
P.W. Rosario, T.A. Fagundes, A.V. Fagundes, A.L. Barraso, L.L. Rezende, E.L. Padrao, V.C. Guimaraes, S. Purisch, Radioiodine therapy and age at menopause in patients with thyroid cancer. Clin. Endocrinol. 64, 225–2266 (2006)
D. Dewailly, C.Y. Andersen, A. Balen, F. Broekmans, N. Dilaver, R. Fanchin, G. Griesinger, T.W. Kelsey, A. La Marca, C. Lambalk, H. Mason, S.M. Nelson, J.A. Visser, W.H. Wallace, R.A. Anderson, The physiology and clinical utility of anti-Mullerian hormone in women. Hum. Reprod. Update 20, 370–385 (2014)
A. Kruszyńska, J. Słowińska-Srzednicka, Anti-Müllerian hormone (AMH) as a good predictor of time of menopause. Menopause Rev. 16, 47–50 (2017)
F. Acıbucu, D.O.Acıbucu, Ö.B.Akkar, H.S.Dokmetas, Evaluation of ovarian reserve with AMH level in patients with well-differentiated thyroid cancer receiving radioactive iodine ablation treatment. Exp. Clin. Endocrinol. Diabetes 124, 593–596 (2016).
D. Gassner, R. Jung, First fully automated immunoassay for anti-Müllerian hormone. Clin. Chem. Lab. Med. 52, 1143–1152 (2014)
M. Giusti, L. Mortara, N. Macchello, E. Monti, G. Pera, M. Marenzana, Utility of a liquid formulation of levo-thyroxine in differentiated thyroid cancer patients. Drug. Res. (Stuttg.). 65, 332–336 (2015)
B.G. Reed, B.R. Carr, in Normal menstrual cycle and the control of ovulation, ed. by L.J De Groot, G. Chrousos, K. Dungan, K.R. Feingold, A. Grossman, J.M. Hershman, C. Koch, M. Korbonits, R. McLachlan, M. New, J. Purnell, R. Rebar, F. Singer, A. Vinik Endotext [Internet]. (MDText.com Inc, South Dartmouth, 2015).
H.M. Chang, C. Klausen, P.C. Leung, Antimüllerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil. Steril. 100, 585–592 (2013)
S.C. Roberts, S.M. Seav, T.W. McDade, S.A. Dominick, J.R. Gorman, B.W. Whitcomb, H.I. Su, Self-collected dried blood spots as a tool for measuring ovarian reserve in young female cancer survivors. Hum. Reprod. 31, 1570–1578 (2016)
C.P. Hagen, S. Vestergaard, A. Juul, N.E. Skakkebæk, A.M. Andersson, K.M. Main, N.,H. Hjøllund, E. Ernst, J.P. Bonde, R.A. Anderson, T.K. Jensen, Low concentration of circulating antimüllerian hormone is not predictive of reduced fecundability in young healthy women: a prospective cohort study. Fertil. Steril. 98, 1602–1608 (2012)
A.Z. Steiner, A.H. Herring, J.S. Kesner, J.W. Meadows, F.Z. Stanczyk, S. Hoberman, D.D. Baird, Antimüllerian hormone as a predictor of natural fecundability in women aged 30-42 years. Obstet. Gynecol. 117, 798–804 (2012)
T.H. Lee, C.H. Liu, C.C. Huang, K.C. Hsieh, P.M. Lin, M.S. Lee, Impact of female age and male infertility on ovarian reserve markers to predict outcome of assisted reproduction technology cycles. Reprod. Biol. Endocrinol. 7, 100 (2009). https://doi.org/10.1186/1477-7827-7-100
R.A. Anderson, E. Anckaert, E. Bosch, D. Dewailly, C.E. Dunlop, D. Fehr, L. Nardo, J. Smitz, K. Tremellen, B. Denk, A. Geistanger, M. Hund, Prospective study into the value of the automated Elecsys antimüllerian hormone assay for the assessment of the ovarian growing follicle pool. Fertil. Steril. 103, 1074–1080 (2015)
J.H. Yoo, H.O. Kim, S.W. Cha, C.W. Park, K.M. Yang, I.O. Song, M.K. Koong, I.S. Kang, Age specific serum anti-Müllerian hormone levels in 1,298 Korean women with regular menstruation. Clin. Exp. Reprod. Med. 38, 93–97 (2011)
J.E. Lee, S.H. Yoon, H.O. Kim, E.G. Min, Correlation between the serum luteinizing hormone to follicle-stimulating hormone ratio and the anti-Müllerian hormone levels in normo-ovulatory women. J. Korean Med. Sci. 30, 296–300 (2015)
N.P. Polyzos, E. Sakkas, A. Vaiarelli, K. Poppe, M. Camus, H. Tournaye, Thyroid autoimmunity, hypothyroidism and ovarian reserve: a cross-sectional study of 5000 women based on age-specific AMH values. Hum. Reprod. 30, 1690–1696 (2015)
F. Magri, L. Schena, V. Capelli, M. Gaiti, F. Zerbini, E. Brambilla, M. Rotondi, M. De Amici, A. Spinillo, R.E. Nappi, L. Chiovato, Anti-Mullerian hormone as a predictor of ovarian reserve in ART protocols: the hidden role of thyroid autoimmunity. Reprod. Biol. Endocrinol. 13, 106 (2015). https://doi.org/10.1186/s12958-015-0103-3
O. Erol, M. Parlak, H.Y. Ellidağ, A.E. Parlak, A.U. Derbent, E. Eren, N. Yılmaz, Serum anti-Müllerian hormone levels in euthyroid adolescent girls with Hashimoto’s thyroiditis: relationship to antioxidant status. Eur. J. Obstet. Gynecol. Reprod. Biol. 203, 204–209 (2016)
A. Weghofer, D.H. Barad, S. Darmon, V.A. Kushnir, N. Gleicher, What affects functional ovarian reserve, thyroid function or thyroid autoimmunity? Reprod. Biol. Endocrinol. 14, 26 (2016). https://doi.org/10.1186/s12958-016-0162-0
D. Unuane, B. Velkeniers, B. Bravenboer, P. Drakopoulos, H. Tournaye, J. Parra, M. De Brucker, Impact of thyroid autoimmunity in euthyroid women on live birth rate after IUI. Hum. Reprod. 32, 915–922 (2017)
K. Kuroda, T. Uchida, S. Nagai, R. Ozaki, T. Yamaguchi, Y. Sato, J.J. Brosens, S. Takeda, Elevated serum thyroid-stimulating hormone is associated with decreased anti-Müllerian hormone in infertile women of reproductive age. J. Assist. Reprod. Genet. 32, 243–247 (2015)
G.E. Krassas, K. Poppe, D. Glinoer, Thyroid function and human reproductive health. Endocr. Rev. 31, 702–755 (2010)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were carried out in accordance with the ethical standards of the institutional and national committees on human experimentation and with the 1975 Helsinki Declaration, as revised in 2008.
Informed consent
Informed consent to inclusion in the study was obtained from all patients.
Rights and permissions
About this article
Cite this article
Giusti, M., Mittica, M., Comite, P. et al. Anti-Müllerian hormone in pre-menopausal females after ablative radioiodine treatment for differentiated thyroid cancer. Endocrine 60, 516–523 (2018). https://doi.org/10.1007/s12020-017-1510-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1510-3