Abstract
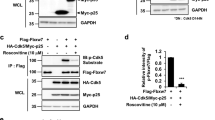
Cyclin-dependent kinase (Cdk) 5 is critical for central nervous system development and neuron-specific functions including neurite outgrowth as well as synaptic function and plasticity. Cdk5 activity requires association with one of the two regulatory subunits, called p35 and p39. p35 redistribution as well as misregulation of Cdk5 activity is followed by cell death in several models of neurodegeneration. Posttranslational protein modification by small ubiquitin-related modifier (SUMO) proteins (sumoylation) has emerged as key regulator of protein targeting and protein/protein interaction. Under cell-free in vitro conditions, we found p35 covalently modified by SUMO1. Using both biochemical and FRET-/FLIM-based approaches, we demonstrated that SUMO2 is robustly conjugated to p35 in cells and identified the two major SUMO acceptor lysines in p35, K246 and K290. Furthermore, different degrees of oxidative stress resulted in differential p35 sumoylation, linking oxidative stress that is encountered in neurodegenerative diseases to the altered activity of Cdk5. Functionally, sumoylation of p35 increased the activity of the p35/Cdk5 complex. We thus identified a novel neuronal SUMO target and show that sumoylation is a likely candidate mechanism for the rapid modulation of p35/Cdk5 activity in physiological situations as well as in disease.





Similar content being viewed by others
References
Amor, S., Peferoen, L. A., Vogel, D. Y., Breur, M., van der Valk, P., Baker, D., et al. (2014). Inflammation in neurodegenerative diseases–an update. Immunology, 142(2), 151–166.
Asada, A., Yamamoto, N., Gohda, M., Saito, T., Hayashi, N., & Hisanaga, S. (2008). Myristoylation of p39 and p35 is a determinant of cytoplasmic or nuclear localization of active cyclin-dependent kinase 5 complexes. Journal of Neurochemistry, 106(3), 1325–1336.
Bernier-Villamor, V., Sampson, D. A., Matunis, M. J., & Lima, C. D. (2002). Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell, 108(3), 345–356.
Bossis, G., & Melchior, F. (2006). Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Molecular Cell, 21(3), 349–357.
Chae, T., Kwon, Y. T., Bronson, R., Dikkes, P., Li, E., & Tsai, L. H. (1997). Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron, 18(1), 29–42.
Choe, E. A., Liao, L., Zhou, J. Y., Cheng, D., Duong, D. M., Jin, P., et al. (2007). Neuronal morphogenesis is regulated by the interplay between cyclin-dependent kinase 5 and the ubiquitin ligase mind bomb 1. Journal of Neuroscience, 27(35), 9503–9512.
Day, R. N., Booker, C. F., & Periasamy, A. (2008). Characterization of an improved donor fluorescent protein for Forster resonance energy transfer microscopy. Journal of Biomedial Optics, 13(3), 031203.
Desterro, J. M., Rodriguez, M. S., Kemp, G. D., & Hay, R. T. (1999). Identification of the enzyme required for activation of the small ubiquitin-like protein SUMO-1. Journal of Biological Chemistry, 274(15), 10618–10624.
Dhavan, R., & Tsai, L. H. (2001). A decade of CDK5. Nature Reviews Molecular Cell Biology, 2(10), 749–759.
Eckermann, K. (2013). SUMO and parkinson’s disease. Neuromolecular Med, 15(4), 737–759.
Feligioni, M., & Nistico, R. (2013). SUMO: a (oxidative) stressed protein. Neuromolecular Medicine, 15(4), 707–719.
Fu, X., Choi, Y. K., Qu, D., Yu, Y., Cheung, N. S., & Qi, R. Z. (2006). Identification of nuclear import mechanisms for the neuronal Cdk5 activator. Journal of Biological Chemistry, 281(51), 39014–39021.
Gill, G. (2004). SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes & Development, 18(17), 2046–2059.
Golebiowski, F., Matic, I., Tatham, M. H., Cole, C., Yin, Y., Nakamura, A., et al. (2009). System-wide changes to SUMO modifications in response to heat shock. Science Signaling, 2(72), ra24.
Gong, L., Millas, S., Maul, G. G., & Yeh, E. T. (2000). Differential regulation of sentrinized proteins by a novel sentrin-specific protease. Journal of Biological Chemistry, 275(5), 3355–3359.
Halliwell, B., Clement, M. V., & Long, L. H. (2000). Hydrogen peroxide in the human body. FEBS Letters, 486(1), 10–13.
Hecker, C. M., Rabiller, M., Haglund, K., Bayer, P., & Dikic, I. (2006). Specification of SUMO1- and SUMO2-interacting motifs. Journal of Biological Chemistry, 281(23), 16117–16127.
Hsiao, K., Bozdagi, O., & Benson, D. L. (2014). Axonal cap-dependent translation regulates presynaptic p35. Developmental Neurobiology, 74(3), 351–364.
Jaffray, E. G., & Hay, R. T. (2006). Detection of modification by ubiquitin-like proteins. Methods, 38(1), 35–38.
Kim, E. T., Kim, K. K., Matunis, M. J., & Ahn, J. H. (2009). Enhanced SUMOylation of proteins containing a SUMO-interacting motif by SUMO-Ubc9 fusion. Biochemical and Biophysical Research Communications, 388(1), 41–45.
Krumova, P., Meulmeester, E., Garrido, M., Tirard, M., Hsiao, H. H., Bossis, G., et al. (2011). Sumoylation inhibits alpha-synuclein aggregation and toxicity. Journal of Cell Biology, 194(1), 49–60.
Krumova, P., & Weishaupt, J. H. (2013). Sumoylation in neurodegenerative diseases. Cellular and Molecular Life Sciences, 70(12), 2123–2138.
Li, Z., David, G., Hung, K. W., DePinho, R. A., Fu, A. K., & Ip, N. Y. (2004). Cdk5/p35 phosphorylates mSds3 and regulates mSds3-mediated repression of transcription. Journal of Biological Chemistry, 279(52), 54438–54444.
Macauley, M. S., Errington, W. J., Scharpf, M., Mackereth, C. D., Blaszczak, A. G., Graves, B. J., et al. (2006). Beads-on-a-string, characterization of ETS-1 sumoylated within its flexible N-terminal sequence. Journal of Biological Chemistry, 281(7), 4164–4172.
Mahajan, R., Delphin, C., Guan, T., Gerace, L., & Melchior, F. (1997). A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell, 88(1), 97–107.
Matunis, M. J., Coutavas, E., & Blobel, G. (1996). A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. Journal of Cell Biology, 135(6 Pt 1), 1457–1470.
Mukhopadhyay, D., & Dasso, M. (2007). Modification in reverse: the SUMO proteases. Trends in Biochemical Sciences, 32(6), 286–295.
Nagai, T., Ibata, K., Park, E. S., Kubota, M., Mikoshiba, K., & Miyawaki, A. (2002). A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nature Biotechnology, 20(1), 87–90.
Nguyen, M. D., Lariviere, R. C., & Julien, J. P. (2001). Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron, 30(1), 135–147.
Nikolic, M., Dudek, H., Kwon, Y. T., Ramos, Y. F., & Tsai, L. H. (1996). The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes & Development, 10(7), 816–825.
O’Hare, M. J., Kushwaha, N., Zhang, Y., Aleyasin, H., Callaghan, S. M., Slack, R. S., et al. (2005). Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. Journal of Neuroscience, 25(39), 8954–8966.
Osuga, H., Osuga, S., Wang, F., Fetni, R., Hogan, M. J., Slack, R. S., et al. (2000). Cyclin-dependent kinases as a therapeutic target for stroke. Proceedings of the National Academy of Sciences USA, 97(18), 10254–10259.
Patrick, G. N., Zhou, P., Kwon, Y. T., Howley, P. M., & Tsai, L. H. (1998). p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway. Journal of Biological Chemistry, 273(37), 24057–24064.
Patrick, G. N., Zukerberg, L., Nikolic, M., de la Monte, S., Dikkes, P., & Tsai, L. H. (1999). Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature, 402(6762), 615–622.
Patzke, H., & Tsai, L. H. (2002). Calpain-mediated cleavage of the cyclin-dependent kinase-5 activator p39 to p29. Journal of Biological Chemistry, 277(10), 8054–8060.
Poon, R. Y., Lew, J., & Hunter, T. (1997). Identification of functional domains in the neuronal Cdk5 activator protein. Journal of Biological Chemistry, 272(9), 5703–5708.
Sahlgren, C. M., Pallari, H. M., He, T., Chou, Y. H., Goldman, R. D., & Eriksson, J. E. (2006). A nestin scaffold links Cdk5/p35 signaling to oxidant-induced cell death. EMBO Journal, 25(20), 4808–4819.
Saitoh, H., & Hinchey, J. (2000). Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. Journal of Biological Chemistry, 275(9), 6252–6258.
Sapetschnig, A., Rischitor, G., Braun, H., Doll, A., Schergaut, M., Melchior, F., et al. (2002). Transcription factor Sp3 is silenced through SUMO modification by PIAS1. EMBO Journal, 21(19), 5206–5215.
Shea, T. B., Zheng, Y. L., Ortiz, D., & Pant, H. C. (2004). Cyclin-dependent kinase 5 increases perikaryal neurofilament phosphorylation and inhibits neurofilament axonal transport in response to oxidative stress. Journal of Neuroscience Research, 76(6), 795–800.
Shin, E. J., Shin, H. M., Nam, E., Kim, W. S., Kim, J. H., Oh, B. H., et al. (2012). DeSUMOylating isopeptidase: a second class of SUMO protease. EMBO Reports, 13(4), 339–346.
Shukla, V., Mishra, S. K., & Pant, H. C. (2011). Oxidative stress in neurodegeneration. Advance Pharmacology Science, 2011, 572634.
Smith, P. D., Crocker, S. J., Jackson-Lewis, V., Jordan-Sciutto, K. L., Hayley, S., Mount, M. P., et al. (2003). Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson’s disease. Proceedings of the National Academy of Sciences USA, 100(23), 13650–13655.
Su, S. C., & Tsai, L. H. (2011). Cyclin-dependent kinases in brain development and disease. Annual Review of Cell and Developmental Biology, 27, 465–491.
Sun, K. H., Chang, K. H., Clawson, S., Ghosh, S., Mirzaei, H., Regnier, F., et al. (2011). Glutathione-S-transferase P1 is a critical regulator of Cdk5 kinase activity. Journal of Neurochemistry, 118(5), 902–914.
Tan, T. C., Valova, V. A., Malladi, C. S., Graham, M. E., Berven, L. A., Jupp, O. J., et al. (2003). Cdk5 is essential for synaptic vesicle endocytosis. Nature Cell Biology, 5(8), 701–710.
Tang, D., Chun, A. C., Zhang, M., & Wang, J. H. (1997). Cyclin-dependent kinase 5 (Cdk5) activation domain of neuronal Cdk5 activator. Evidence of the existence of cyclin fold in neuronal Cdk5a activator. Journal of Biological Chemistry, 272(19), 12318–12327.
Tang, D., Yeung, J., Lee, K. Y., Matsushita, M., Matsui, H., Tomizawa, K., et al. (1995). An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. Journal of Biological Chemistry, 270(45), 26897–26903.
Tarricone, C., Dhavan, R., Peng, J., Areces, L. B., Tsai, L. H., & Musacchio, A. (2001). Structure and regulation of the CDK5-p25(nck5a) complex. Molecular Cell, 8(3), 657–669.
Tatham, M. H., Jaffray, E., Vaughan, O. A., Desterro, J. M., Botting, C. H., Naismith, J. H., et al. (2001). Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. Journal of Biological Chemistry, 276(38), 35368–35374.
Tsai, L. H., Delalle, I., Caviness, V. S, Jr, Chae, T., & Harlow, E. (1994). p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature, 371(6496), 419–423.
van den Heuvel, S., & Harlow, E. (1993). Distinct roles for cyclin-dependent kinases in cell cycle control. Science, 262(5142), 2050–2054.
Verstegen, A. M., Tagliatti, E., Lignani, G., Marte, A., Stolero, T., Atias, M., et al. (2014). Phosphorylation of synapsin I by cyclin-dependent kinase-5 sets the ratio between the resting and recycling pools of synaptic vesicles at hippocampal synapses. Journal of Neuroscience, 34(21), 7266–7280.
Weishaupt, J. H., Kussmaul, L., Grotsch, P., Heckel, A., Rohde, G., Romig, H., et al. (2003). Inhibition of CDK5 is protective in necrotic and apoptotic paradigms of neuronal cell death and prevents mitochondrial dysfunction. Molecular and Cellular Neuroscience, 24(2), 489–502.
Wittmann, C., Chockley, P., Singh, S. K., Pase, L., Lieschke, G. J., & Grabher, C. (2012). Hydrogen peroxide in inflammation: messenger, guide, and assassin. Advances in Hematology, 2012, 541471.
Zukerberg, L. R., Patrick, G. N., Nikolic, M., Humbert, S., Wu, C. L., Lanier, L. M., et al. (2000). Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron, 26(3), 633–646.
Acknowledgments
We thank Christine Poser and Claudia Fokken for excellent technical support. We thank Ron Hay (University of Dundee, Dundee, UK) for providing us with the His6-SUMO1 and His6-SUMO2 plasmids, Frauke Melchior (ZMBH, Heidelberg, Germany) for the components used for the in vitro sumoylation assay and Gertrude Bunt (University Medical Center Göttingen, Göttingen, Germany) for mVenus and mTFP plasmids. This work was supported by the Cluster of Excellence and DFG Research Center Nanoscale Microscopy and Molecular Physiology of the Brain (CNMPB).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Anja Büchner, Petranka Krumova, Katrin Eckermann, and Jochen H. Weishaupt have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Büchner, A., Krumova, P., Ganesan, S. et al. Sumoylation of p35 Modulates p35/Cyclin-Dependent Kinase (Cdk) 5 Complex Activity. Neuromol Med 17, 12–23 (2015). https://doi.org/10.1007/s12017-014-8336-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-014-8336-4