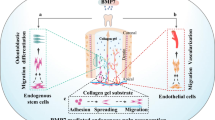
Abstract
Deep caries, trauma, and severe periodontitis result in pulpitis, pulp necrosis, and eventually pulp loss. However, no clinical therapy can regenerate lost pulp. A novel pulp regeneration strategy for clinical application is urgently needed. Signaling transduction plays an essential role in regulating the regenerative potentials of dental stem cells. Cytokines or growth factors, such as stromal cell-derived factor (SDF), fibroblast growth factor (FGF), bone morphogenetic protein (BMP), vascular endothelial growth factor (VEGF), WNT, can promote the migration, proliferation, odontogenic differentiation, pro-angiogenesis, and pro-neurogenesis potentials of dental stem cells respectively. Using the methods of signaling modulation including growth factors delivery, genetic modification, and physical stimulation has been applied in multiple preclinical studies of pulp regeneration based on cell transplantation or cell homing. Transplanting dental stem cells and growth factors encapsulated into scaffold regenerated vascularized pulp-like tissue in the root canal. Also, injecting a flowable scaffold only with chemokines recruited endogenous stem/progenitor cells for pulp regeneration. Notably, dental pulp regeneration has gradually developed into the clinical phase. These findings enlightened us on a novel strategy for structural and functional pulp regeneration through elaborate modulation of signaling transduction spatially and temporally via clinically applicable growth factors delivery. But challenges, such as the adverse effects of unphysiological signaling activation, the controlled drug release system, and the safety of gene modulation, are necessary to be tested in future works for promoting the clinical translation of pulp regeneration.
Graphical Abstract





Similar content being viewed by others
Data Availability
Not applicable.
References
Itoh, I., et al. (1987). [Relationships among the tooth crown, the root structure and the pulp cavity in human molars]. Shikwa Gakuho, 87(3), 529–538.
Kleinert, A., et al. (2018). Endodontium - together or separately? Folia Morphologica (Warsz), 77(3), 409–415. https://doi.org/10.5603/FM.a2018.0008.
Gronthos, S., et al. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America, 97(25), 13625–13630. https://doi.org/10.1073/pnas.240309797.
Zhu, L., et al. (2019). Dental pulp stem cells overexpressing stromal-derived factor-1alpha and vascular endothelial growth factor in dental pulp regeneration. Clinical Oral Investigations, 23(5), 2497–2509. https://doi.org/10.1007/s00784-018-2699-0.
Kim, J. Y., et al. (2010). Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Engineering. Part A, 16(10), 3023–3031. https://doi.org/10.1089/ten.TEA.2010.0181.
Gronthos, S., et al. (2002). Stem cell properties of human dental pulp stem cells. Journal of Dental Research, 81(8), 531–535. https://doi.org/10.1177/154405910208100806.
Miura, M., et al. (2003). SHED: stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences of the United States of America, 100(10), 5807–5812. https://doi.org/10.1073/pnas.0937635100.
Guo, L., et al. (2013). Comparison of odontogenic differentiation of human dental follicle cells and human dental papilla cells. PLoS One, 8(4), e62332. doi:https://doi.org/10.1371/journal.pone.0062332.
Lei, M., et al. (2014). Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials, 35(24), 6332–6343. doi:https://doi.org/10.1016/j.biomaterials.2014.04.071.
Nowicka, A., et al. (2013). Response of human dental pulp capped with biodentine and mineral trioxide aggregate. Journal of Endodontia, 39(6), 743–747. https://doi.org/10.1016/j.joen.2013.01.005.
Yoshida, S., et al. (2016). Semaphorin 3A induces odontoblastic phenotype in dental pulp stem cells. Journal of Dental Research, 95(11), 1282–1290. https://doi.org/10.1177/0022034516653085.
Iohara, K., et al. (2013). A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem Cells Translational Medicine, 2(7), 521–533. https://doi.org/10.5966/sctm.2012-0132.
Zhu, X., et al. (2018). A miniature swine model for stem cell-based de novo regeneration of dental pulp and dentin-like tissue. Tissue Engineering. Part C, Methods, 24(2), 108–120. https://doi.org/10.1089/ten.tec.2017.0342.
Iohara, K., et al. (2018). Allogeneic transplantation of mobilized dental pulp stem cells with the mismatched dog leukocyte antigen type is safe and efficacious for total pulp regeneration. Stem Cell Research & Therapy, 9(1), 116. https://doi.org/10.1186/s13287-018-0855-8.
Hilkens, P., et al. (2017). The angiogenic potential of DPSCs and SCAPs in an in vivo model of dental pulp regeneration. Stem Cells International, 2017, 2582080. https://doi.org/10.1155/2017/2582080.
Itoh, Y., et al. (2018). Pulp regeneration by 3-dimensional dental pulp stem cell constructs. Journal of Dental Research, 97(10), 1137–1143. https://doi.org/10.1177/0022034518772260.
Nakashima, M., et al. (2017). Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Research & Therapy, 8(1), 61. https://doi.org/10.1186/s13287-017-0506-5.
Xuan, K., et al. (2018). Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Science Translational Medicine, 10(455). https://doi.org/10.1126/scitranslmed.aaf3227.
Sabbagh, J., et al. (2020). Differences in osteogenic and odontogenic differentiation potential of DPSCs and SHED. Journal of Dentistry, 101, 103413. https://doi.org/10.1016/j.jdent.2020.103413.
Shi, S., et al. (2005). The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthodontics & Craniofacial Research, 8(3), 191–199. https://doi.org/10.1111/j.1601-6343.2005.00331.x.
Cordeiro, M. M., et al. (2008). Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. Journal of Endodontia, 34(8), 962–969. https://doi.org/10.1016/j.joen.2008.04.009.
Rosa, V., et al. (2013). Dental pulp tissue engineering in full-length human root canals. Journal of Dental Research, 92(11), 970–975. https://doi.org/10.1177/0022034513505772.
Yoo, Y. J., et al. (2016). Regenerative characteristics of apical papilla-derived cells from immature teeth with pulpal and periapical pathosis. Journal of Endodontia, 42(11), 1626–1632. https://doi.org/10.1016/j.joen.2016.08.004.
Zhang, H., et al. (2015). Canonical Wnt signaling acts synergistically on BMP9-induced osteo/odontoblastic differentiation of stem cells of dental apical papilla (SCAPs). Biomaterials, 39, 145–154. doi:https://doi.org/10.1016/j.biomaterials.2014.11.007.
Na, S., et al. (2016). Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. Journal of Tissue Engineering and Regenerative Medicine, 10(3), 261–270. https://doi.org/10.1002/term.1686.
Liu, J. Y., et al. (2015). CXC Chemokine Receptor 4 Is Expressed Paravascularly in Apical Papilla and Coordinates with Stromal Cell-derived Factor-1alpha during Transmigration of Stem Cells from Apical Papilla. J Endod, 41(9), 1430–1436. doi:https://doi.org/10.1016/j.joen.2015.04.006.
Yu, J. C., et al. (2015). Hedgehog signaling regulates dental papilla formation and tooth size during zebrafish odontogenesis. Developmental Dynamics, 244(4), 577–590. https://doi.org/10.1002/dvdy.24258.
Liu, C., et al. (2013). FGF signaling sustains the odontogenic fate of dental mesenchyme by suppressing beta-catenin signaling. Development, 140(21), 4375–4385. doi:https://doi.org/10.1242/dev.097733.
Shi, C., et al. (2019). BMP signaling in regulating mesenchymal stem cells in incisor homeostasis. Journal of Dental Research, 98(8), 904–911. https://doi.org/10.1177/0022034519850812.
Li, D., et al. (2014). The effects of LPS on adhesion and migration of human dental pulp stem cells in vitro. Journal of Dentistry, 42(10), 1327–1334. https://doi.org/10.1016/j.jdent.2014.07.007.
Simon, S., et al. (2010). The MAP kinase pathway is involved in odontoblast stimulation via p38 phosphorylation. Journal of Endodontia, 36(2), 256–259. https://doi.org/10.1016/j.joen.2009.09.019.
Smith, A. J., et al. (2001). Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Critical Reviews in Oral Biology and Medicine, 12(5), 425–437. https://doi.org/10.1177/10454411010120050501.
Suzuki, T., et al. (2011). Induced migration of dental pulp stem cells for in vivo pulp regeneration. Journal of Dental Research, 90(8), 1013–1018. https://doi.org/10.1177/0022034511408426.
Yang, J. W., et al. (2015). Autophagy in SDF-1alpha-mediated DPSC migration and pulp regeneration. Biomaterials, 44, 11–23. doi:https://doi.org/10.1016/j.biomaterials.2014.12.006.
Xiao, M., et al. (2019). Stromal-derived Factor-1alpha signaling is involved in bone morphogenetic protein-2-induced odontogenic differentiation of stem cells from apical papilla via the Smad and Erk signaling pathways. Experimental Cell Research, 381(1), 39–49. https://doi.org/10.1016/j.yexcr.2019.04.036.
Hayashi, Y., et al. (2015). CXCL14 and MCP1 are potent trophic factors associated with cell migration and angiogenesis leading to higher regenerative potential of dental pulp side population cells. Current Stem Cell Research & Therapy, 6, 111. https://doi.org/10.1186/s13287-015-0088-z.
Li, M., et al. (2017). SDF-1/CXCR4 axis induces human dental pulp stem cell migration through FAK/PI3K/Akt and GSK3beta/beta-catenin pathways. Scientific Reports, 7, 40161. https://doi.org/10.1038/srep40161.
Pan, S., et al. (2013). SCF promotes dental pulp progenitor migration, neovascularization, and collagen remodeling - potential applications as a homing factor in dental pulp regeneration. Stem Cell Reviews and Reports, 9(5), 655–667. https://doi.org/10.1007/s12015-013-9442-7.
Kim, D. S., et al. (2014). Effects of glutamine on proliferation, migration, and differentiation of human dental pulp cells. Journal of Endodontia, 40(8), 1087–1094. https://doi.org/10.1016/j.joen.2013.11.023.
Kim, J., et al. (2014). Treatment of FGF-2 on stem cells from inflamed dental pulp tissue from human deciduous teeth. Oral Diseases, 20(2), 191–204. https://doi.org/10.1111/odi.12089.
He, H., et al. (2008). Effects of FGF2 and TGFbeta1 on the differentiation of human dental pulp stem cells in vitro. Cell Biology International, 32(7), 827–834. https://doi.org/10.1016/j.cellbi.2008.03.013.
Chang, Y. C., et al. (2017). Basic fibroblast growth factor regulates gene and protein expression related to proliferation, differentiation, and matrix production of human dental pulp cells. Journal of Endodontia, 43(6), 936–942. https://doi.org/10.1016/j.joen.2017.01.024.
Zhang, Z., et al. (2014). Effects of WNT10A on proliferation and differentiation of human dental pulp cells. Journal of Endodontia, 40(10), 1593–1599. https://doi.org/10.1016/j.joen.2014.07.009.
Liu, Y., et al. (2013). Down-regulation of Wnt10a affects odontogenesis and proliferation in mesenchymal cells. Biochemical and Biophysical Research Communications, 434(4), 717–721. https://doi.org/10.1016/j.bbrc.2013.03.088.
Zhang, X., et al. (2019). Stathmin regulates the proliferation and odontoblastic/osteogenic differentiation of human dental pulp stem cells through Wnt/beta-catenin signaling pathway. Journal of Proteomics, 202, 103364. https://doi.org/10.1016/j.jprot.2019.04.014.
Lew, W. Z., et al. (2018). Static magnetic fields enhance dental pulp stem cell proliferation by activating the p38 mitogen-activated protein kinase pathway as its putative mechanism. Journal of Tissue Engineering and Regenerative Medicine, 12(1), 19–29. https://doi.org/10.1002/term.2333.
Xia, L., et al. (2013). Enhanced dentin-like mineralized tissue formation by AdShh-transfected human dental pulp cells and porous calcium phosphate cement. PLoS One, 8(5), e62645. doi:https://doi.org/10.1371/journal.pone.0062645.
Qin, W., et al. (2012). Smad 1/5 is involved in bone morphogenetic protein-2-induced odontoblastic differentiation in human dental pulp cells. Journal of Endodontia, 38(1), 66–71. https://doi.org/10.1016/j.joen.2011.09.025.
Yuan, X., et al. (2019). Ciliary IFT80 regulates dental pulp stem cells differentiation by FGF/FGFR1 and Hh/BMP2 signaling. International Journal of Biological Sciences, 15(10), 2087–2099. https://doi.org/10.7150/ijbs.27231.
Woo, S. M., et al. (2016). Combination of mineral trioxide aggregate and platelet-rich fibrin promotes the odontoblastic differentiation and mineralization of human dental pulp cells via BMP/Smad signaling pathway. Journal of Endodontia, 42(1), 82–88. https://doi.org/10.1016/j.joen.2015.06.019.
Li, S., et al. (2015). Extracellular Ca2 + promotes odontoblastic differentiation of dental pulp stem cells via BMP2-mediated Smad1/5/8 and Erk1/2 pathways. Journal of Cellular Physiology, 230(9), 2164–2173. https://doi.org/10.1002/jcp.24945.
Kong, Y., et al. (2019). Magnesium-enriched microenvironment promotes odontogenic differentiation in human dental pulp stem cells by activating ERK/BMP2/Smads signaling. Stem Cell Research & Therapy, 10(1), 378. https://doi.org/10.1186/s13287-019-1493-5.
Wang, J., et al. (2012). Effects of Wnt/beta-catenin signalling on proliferation and differentiation of apical papilla stem cells. Cell Proliferation, 45(2), 121–131. https://doi.org/10.1111/j.1365-2184.2012.00806.x.
Chen, Y. W., et al. (2016). The ionic products from mineral trioxide aggregate-induced odontogenic differentiation of dental pulp cells via activation of the Wnt/beta-catenin signaling pathway. Journal of Endodontia, 42(7), 1062–1069. https://doi.org/10.1016/j.joen.2016.04.019.
Liu, N., et al. (2018). Stiffness regulates the proliferation and osteogenic/odontogenic differentiation of human dental pulp stem cells via the WNT signalling pathway. Cell Proliferation, 51(2), e12435. https://doi.org/10.1111/cpr.12435.
Rahman, S. U., et al. (2018). Fibrous topography-potentiated canonical Wnt signaling directs the odontoblastic differentiation of dental pulp-derived stem cells. ACS Applied Materials & Interfaces, 10(21), 17526–17541. https://doi.org/10.1021/acsami.7b19782.
Kim, Y. S., et al. (2010). Effects of fibroblast growth factor-2 on the expression and regulation of chemokines in human dental pulp cells. Journal of Endodontia, 36(11), 1824–1830. https://doi.org/10.1016/j.joen.2010.08.020.
He, W., et al. (2015). LPS promote the odontoblastic differentiation of human dental pulp stem cells via MAPK signaling pathway. Journal of Cellular Physiology, 230(3), 554–561. https://doi.org/10.1002/jcp.24732.
Cui, D., et al. (2019). Epiregulin enhances odontoblastic differentiation of dental pulp stem cells via activating MAPK signalling pathway. Cell Proliferation, 52(6), e12680. https://doi.org/10.1111/cpr.12680.
Ge, X., et al. (2020). Parathyroid hormone enhances the osteo/odontogenic differentiation of dental pulp stem cells via ERK and P38 MAPK pathways. Journal of Cellular Physiology, 235(2), 1209–1221. https://doi.org/10.1002/jcp.29034.
Zhang, H., et al. (2012). Natural mineralized scaffolds promote the dentinogenic potential of dental pulp stem cells via the mitogen-activated protein kinase signaling pathway. Tissue Engineering. Part A, 18(7–8), 677–691. https://doi.org/10.1089/ten.TEA.2011.0269.
Huang, C. C., et al. (2016). Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials, 111, 103–115. doi:https://doi.org/10.1016/j.biomaterials.2016.09.029.
Holderfield, M. T., et al. (2008). Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-β in vascular morphogenesis. Circulation Research, 102(6), 637–652. https://doi.org/10.1161/circresaha.107.167171.
Janebodin, K., et al. (2013). VEGFR2-dependent angiogenic capacity of pericyte-like dental pulp stem cells. Journal of Dental Research, 92(6), 524–531. https://doi.org/10.1177/0022034513485599.
Kim, M. K., et al. (2014). Hinokitiol increases the angiogenic potential of dental pulp cells through ERK and p38MAPK activation and hypoxia-inducible factor-1alpha (HIF-1alpha) upregulation. Archives of Oral Biology, 59(2), 102–110. https://doi.org/10.1016/j.archoralbio.2013.10.009.
Huang, S. C., et al. (2015). Role of the p38 pathway in mineral trioxide aggregate-induced cell viability and angiogenesis-related proteins of dental pulp cell in vitro. International Endodontic Journal, 48(3), 236–245. https://doi.org/10.1111/iej.12305.
Limjeerajarus, C. N., et al. (2014). Iloprost up-regulates vascular endothelial growth factor expression in human dental pulp cells in vitro and enhances pulpal blood flow in vivo. Journal of Endodontia, 40(7), 925–930. https://doi.org/10.1016/j.joen.2013.10.025.
Shin, M. R., et al. (2015). TNF-alpha and LPS activate angiogenesis via VEGF and SIRT1 signalling in human dental pulp cells. International Endodontic Journal, 48(7), 705–716. https://doi.org/10.1111/iej.12396.
Gong, T., et al. (2019). EphrinB2/EphB4 signaling regulates DPSCs to induce sprouting angiogenesis of endothelial cells. Journal of Dental Research, 98(7), 803–812. https://doi.org/10.1177/0022034519843886.
Yuan, C., et al. (2015). Coculture of stem cells from apical papilla and human umbilical vein endothelial cell under hypoxia increases the formation of three-dimensional vessel-like structures in vitro. Tissue Engineering. Part A, 21(5–6), 1163–1172. https://doi.org/10.1089/ten.TEA.2014.0058.
Bento, L. W., et al. (2013). Endothelial differentiation of SHED requires MEK1/ERK signaling. Journal of Dental Research, 92(1), 51–57. https://doi.org/10.1177/0022034512466263.
Sakai, V. T., et al. (2010). SHED differentiate into functional odontoblasts and endothelium. Journal of Dental Research, 89(8), 791–796. https://doi.org/10.1177/0022034510368647.
Zou, T., et al. (2019). Sema4D/PlexinB1 promotes endothelial differentiation of dental pulp stem cells via activation of AKT and ERK1/2 signaling. Journal of Cellular Biochemistry, 120(8), 13614–13624. https://doi.org/10.1002/jcb.28635.
Zhang, Z., et al. (2016). Wnt/beta-catenin signaling determines the vasculogenic fate of postnatal mesenchymal stem cells. Stem Cells, 34(6), 1576–1587. https://doi.org/10.1002/stem.2334.
Liu, A. Q., et al. (2020). Sensory nerve-deficient microenvironment impairs tooth homeostasis by inducing apoptosis of dental pulp stem cells. Cell Proliferation, 53(5), e12803. https://doi.org/10.1111/cpr.12803.
Pisciotta, A., et al. (2020). Neural crest derived stem cells from dental pulp and tooth-associated stem cells for peripheral nerve regeneration. Neural Regeneration Research, 15(3), 373–381. https://doi.org/10.4103/1673-5374.266043.
Mitsiadis, T. A., et al. (2017). Nerve growth factor signalling in pathology and regeneration of human teeth. Scientific Reports, 7(1), 1327. https://doi.org/10.1038/s41598-017-01455-3.
Kolar, M. K., et al. (2017). The neurotrophic effects of different human dental mesenchymal stem cells. Scientific Reports, 7(1), 12605. https://doi.org/10.1038/s41598-017-12969-1.
Nagashima, K., et al. (2017). Priming with FGF2 stimulates human dental pulp cells to promote axonal regeneration and locomotor function recovery after spinal cord injury. Scientific Reports, 7(1), 13500. https://doi.org/10.1038/s41598-017-13373-5.
Osathanon, T., et al. (2011). Basic fibroblast growth factor inhibits mineralization but induces neuronal differentiation by human dental pulp stem cells through a FGFR and PLCgamma signaling pathway. Journal of Cellular Biochemistry, 112(7), 1807–1816. https://doi.org/10.1002/jcb.23097.
Zhang, J., et al. (2017). Effects of nerve growth factor and basic fibroblast growth factor promote human dental pulp stem cells to neural differentiation. Neurochemical Research, 42(4), 1015–1025. https://doi.org/10.1007/s11064-016-2134-3.
Zhang, J., et al. (2016). Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: potential roles for spinal cord injury therapy. Cell and Tissue Research, 366(1), 129–142. https://doi.org/10.1007/s00441-016-2402-1.
Silva, G. O., et al. (2017). Lipoprotein receptor-related protein 6 signaling is necessary for vasculogenic differentiation of human dental pulp stem cells. Journal of Endodontia, 43(9S), S25–S30. https://doi.org/10.1016/j.joen.2017.06.006.
He, L., et al. (2019). Parenchymal and stromal tissue regeneration of tooth organ by pivotal signals reinstated in decellularized matrix. Nature Materials, 18(6), 627–637. https://doi.org/10.1038/s41563-019-0368-6.
Yang, J., et al. (2015). Bone morphogenetic protein 2-induced human dental pulp cell differentiation involves p38 mitogen-activated protein kinase-activated canonical WNT pathway. International Journal of Oral Science, 7(2), 95–102. https://doi.org/10.1038/ijos.2015.7.
Sagomonyants, K., et al. (2015). Enhanced dentinogenesis of pulp progenitors by early exposure to FGF2. Journal of Dental Research, 94(11), 1582–1590. https://doi.org/10.1177/0022034515599768.
Xu, J. G., et al. (2018). Inhibition of TGF-beta Signaling in SHED enhances endothelial differentiation. Journal of Dental Research, 97(2), 218–225. https://doi.org/10.1177/0022034517733741.
Zaugg, L. K., et al. (2020). Translation approach for dentine regeneration using GSK-3 antagonists. Journal of Dental Research, 22034520908593. https://doi.org/10.1177/0022034520908593.
Ali, M., et al. (2019). Lithium-containing surface pre-reacted glass fillers enhance hDPSC functions and induce reparative dentin formation in a rat pulp capping model through activation of Wnt/β-catenin signaling. Acta Biomaterialia, 96, 594–604. doi:https://doi.org/10.1016/j.actbio.2019.06.016.
Cockrell, A. S., et al. (2007). Gene delivery by lentivirus vectors. Molecular Biotechnology, 36(3), 184–204. https://doi.org/10.1007/s12033-007-0010-8.
Zhang, M., et al. (2017). The effects of platelet-derived growth factor-BB on human dental pulp stem cells mediated dentin-pulp complex regeneration. Stem Cells Translational Medicine, 6(12), 2126–2134. https://doi.org/10.1002/sctm.17-0033.
He, F., et al. (2009). Effects of Notch ligand Delta1 on the proliferation and differentiation of human dental pulp stem cells in vitro. Archives of Oral Biology, 54(3), 216–222. https://doi.org/10.1016/j.archoralbio.2008.10.003.
Yun, H. M., et al. (2016). Magnetic nanofiber scaffold-induced stimulation of odontogenesis and pro-angiogenesis of human dental pulp cells through Wnt/MAPK/NF-kappaB pathways. Dental Materials, 32(11), 1301–1311. https://doi.org/10.1016/j.dental.2016.06.016.
Scheven, B. A., et al. (2009). VEGF and odontoblast-like cells: stimulation by low frequency ultrasound. Archives of Oral Biology, 54(2), 185–191. https://doi.org/10.1016/j.archoralbio.2008.09.008.
Eramo, S., et al. (2018). Dental pulp regeneration via cell homing. International Endodontic Journal, 51(4), 405–419. https://doi.org/10.1111/iej.12868.
Nakashima, M., et al. (2011). Regeneration of dental pulp by stem cells. Advances in Dental Research, 23(3), 313–319. https://doi.org/10.1177/0022034511405323.
Qiao, J., et al. (2017). Quantification of growth factors in different platelet concentrates. Platelets, 28(8), 774–778. doi:https://doi.org/10.1080/09537104.2016.1267338.
Zhu, X., et al. (2012). Transplantation of dental pulp stem cells and platelet-rich plasma for pulp regeneration. Journal of Endodontia, 38(12), 1604–1609. https://doi.org/10.1016/j.joen.2012.09.001.
Torabinejad, M., et al. (2015). Histologic examination of teeth with necrotic pulps and periapical lesions treated with 2 scaffolds: an animal investigation. Journal of Endodontia, 41(6), 846–852. https://doi.org/10.1016/j.joen.2015.01.026.
Torabinejad, M., et al. (2011). Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: a case report. Journal of Endodontia, 37(2), 265–268. https://doi.org/10.1016/j.joen.2010.11.004.
Dave, J. R., et al. (2018). Dental tissue-derived mesenchymal stem cells: applications in tissue engineering. Critical Reviews in Biomedical Engineering, 46(5), 429–468. https://doi.org/10.1615/CritRevBiomedEng.2018027342.
Shi, X., et al. (2020). Concise review: Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Translational Medicine. https://doi.org/10.1002/sctm.19-0398.
Wang, J., et al. (2020). Retinoic acid signal negatively regulates osteo/odontogenic differentiation of dental pulp stem cells. Stem Cells International, 2020, 1–12. https://doi.org/10.1155/2020/5891783.
Galler, K. M., et al. (2012). A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Engineering. Part A, 18(1–2), 176–184. https://doi.org/10.1089/ten.TEA.2011.0222.
Abasalizadeh, F., et al. (2020). Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. Journal of Biological Engineering, 14, 8. https://doi.org/10.1186/s13036-020-0227-7.
Zhang, B., et al. (2018). Surface-decorated hydroxyapatite scaffold with on-demand delivery of dexamethasone and stromal cell derived factor-1 for enhanced osteogenesis. Materials Science & Engineering. C, Materials for Biological Applications, 89, 355–370. https://doi.org/10.1016/j.msec.2018.04.008.
Skop, N. B., et al. (2013). Heparin crosslinked chitosan microspheres for the delivery of neural stem cells and growth factors for central nervous system repair. Acta Biomaterialia, 9(6), 6834–6843. https://doi.org/10.1016/j.actbio.2013.02.043.
Fukushima, K. A., et al. (2019). Screening of hydrogel-based scaffolds for dental pulp regeneration-A systematic review. Archives of Oral Biology, 98, 182–194. https://doi.org/10.1016/j.archoralbio.2018.11.023.
Ma, L., et al. (2019). Maintained properties of aged dental pulp stem cells for superior periodontal tissue regeneration. Aging and Disease, 10(4), 793–806. https://doi.org/10.14336/AD.2018.0729.
Wang, X., et al. (2014). Promotion of dentin regeneration via CCN3 modulation on Notch and BMP signaling pathways. Biomaterials, 35(9), 2720–2729. doi:https://doi.org/10.1016/j.biomaterials.2013.12.029.
Funding
This work was supported by grants from the National Key Research and Development Program of China (2017YFA0104800), the Fundamental Research Funds for the Central Universities (YJ201878), Technology Innovation Research and Development Project of Chengdu (2019-YF05-00705-SN), Key Project of Sichuan province (2019YFS0311, 2019YFS0515), and the Nature Science Foundation of China (81600912, 31601113).
Author information
Authors and Affiliations
Contributions
CL contributed to the idea for this article and performed the literature search and original draft preparation. LL contributed to the idea for this article, draft preparation, and revision of the manuscript. WDT critically revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Ethics Approval
Not applicable.
Consent for Publication
Not applicable.
Consent to Participate
Not applicable.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, C., Liao, L. & Tian, W. Stem Cell‐based Dental Pulp Regeneration: Insights From Signaling Pathways. Stem Cell Rev and Rep 17, 1251–1263 (2021). https://doi.org/10.1007/s12015-020-10117-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10117-3