Abstract
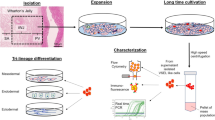
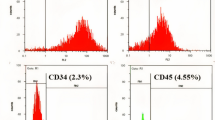
Intercellular communication is one of the most important events in cell population behavior. In the last decade, tunneling nanotubes (TNTs) have been recognized as a new form of long distance intercellular connection. TNT function is to allow molecular and subcellular structure exchange between neighboring cells via the transfer of molecules and organelles such as calcium ions, prions, viral and bacterial pathogens, small lysosomes and mitochondria. New findings support the concept that mesenchymal stem cells (MSCs) can affect cell microenvironment by the release of soluble factors or the transfer of cellular components to neighboring cells, in a way which significantly contributes to cell regulation and tissue repair, although the underlying mechanisms remain poorly understood. MSCs have many advantages for their implementation in regenerative medicine. The TNTs in these cell types are heterogeneous in both structure and function, probably due to their highly dynamic behavior. In this work we report an extensive and detailed description of types, structure, components, dynamics and functionality of the TNTs bridging neighboring human umbilical cord MSCs obtained from Wharton”s jelly. Characterization studies were carried out through phase contrast, fluorescence, electron microscopy and time lapse images with the aim of describing cells suitable for an eventual regenerative medicine.






Similar content being viewed by others
References
Rustom, A., Saffrich, R., Markovic, I., Walther, P., & Gerdes, H. H. (2004). Nanotubular highways for intercellular organelle transport. Science, 303, 1007–1010.
Watkins, S. C., & Salter, R. D. (2005). Functional connectivity between immune cells mediated by tunneling nanotubes. Immunity, 23, 309–318.
Koyanagi, M., Brandes, R. P., Haendeler, J., Zeiher, A. M., & Dimmeler, S. (2005). Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circulation Research, 96, 1039–1041.
Önfelt, B., Nedvetzki, S., Benninger, R. K., Purbhoo, M. A., Sowinski, S., Hume, A. N., Seabra, M. C., Neil, M. A., French, P. M., & Davis, D. M. (2006). Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. Journal of Immunology, 177, 8476–8483.
Plotnikov, E. Y., Khryapenkov, T. G., Galkina, S., Sukhikhb, G., & Zorova, D. (2010). Cytoplasm and organelletransfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Experimental Cell Research, 316, 2447–2455.
Gerdes, H. H., & Carvalho, R. (2008). Intercellular transfer mediated by tunneling nanotubes. Current Opinion in Cell Biology, 20, 470–475.
Sisakhtnezhad, S., & Khosravi, L. (2015). Emerging physiological and pathological implications of tunneling nanotubes formation between cells. European Journal of Cell Biology, 94, 429–443.
Gerdes, H., Bukoreshtliev, N., & Barroso, J. (2007). Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Letters, 581, 2194–2201.
Yasuda, K., Khandare, A., Burianovsky, L., Maruyama, S., Zhang, F., Nasjletti, A., & Goligorsky, M. (2011). Tunneling nanotubes mediate rescue of prematurely senescent endothelial cells by endothelial progenitors: exchange of lysosomal pool. Aging, 3, 597–608.
Carvalho, M., Teixeira, F., Reis, R., Sousa, N., & Salgado, A. (2011). Mesenchymal stem cells in the umbilical cord: phenotypic characterization, secretome and applications in central nervous system regenerative medicine. Current Stem Cell Research & Therapy, 6, 221–228.
Batsali, A. K., Kastrinaki, M. C., Papadaki, H. A., & Pontikoglou, C. (2013). Mesenchymal stem cells derived from Wharton's jelly of the umbilical cord: biological properties and emerging clinical applications. Current Stem Cell Research & Therapy, 8, 144–155.
Luzzani, C., Neiman, G., Garate, X., Questa, M., Solari, C., Fernandez-Espinosa, D., García, M., Errecalde, A., Guberman, A., Scassa, M. E., Sevlever, G. E., Romorini, L., & Miriuka, S. G. (2015). A therapy-grade protocol for differentiation of pluripotent stem cells into mesenchymal stem cells using platelet lysate as supplement. Stem Cell Research & Therapy, 6, 6 .http://stemcellres.com/content/6/1/6
Noble, C., Nilsson, A., Freitag, C., Beech, J., Tegenfeldt, J., & Ambjörnsson, T. (2015). A fast and scalable kymograph alignment algorithm for nanochannel-based optical DNA mappings. PloS One. doi:10.1371/journal.pone.0121905.
Thayanithy, V., Dickson, E. L., Steer, C., Subramanian, S., & Lou, E. (2014). Tumor-stromal cross talk: direct cell-to-cell transfer of oncogenic microRNAs via tunneling nanotubes. Translational Research, 164, 359–365.
Han, H., Hu, J., Yan, Q., Zhu, J., Zhu, Z., Chen, Y., Sun, J., & Zhang, R. (2016). Bone marrow derived mesenchymal stem cells rescue injured H9c2 cells via transferring intact mitochondria through tunneling nanotubes in an in vitro simulated ischemia/reperfusion model. Molecular Medicine Reports, 13, 1517–1524.
Jackson, M., Morrison, T., Doherty, D., Mc Auley, D., Matthay, M., Kissenpfennig, A., O’Kane, C., & Krasnodembskaya, A. (2016). Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells, 34, 2210–2223.
Wang, H., Hung, S., Peng, S., Huang, C., Wei, H.-M., Guo, Y.-J., et al. (2004). Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells, 22, 1330–1337.
Zani, B., & Edelman, E. (2010). Cellular bridges. Routes for intercellular communication and cell migration. Communicative & Integrative Biology, 3, 215–220.
Veranic, P., Lokar, M., Schütz, G., Weghuber, J., Wieser, S., Hägerstrand, H., et al. (2008). Different types of cell-to-cell connections mediated by nanotubular structures. Biophysical Journal, 95, 4416–4425.
He, K., Shi, X., Zhang, X., Dang, S., Ma, X., Liu, F., et al. (2011). Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovascular Research, 92, 39–47.
Plotnikov, E., Khryapenkova, T., Vasileva, A., Marey, M., Galkina, S., Isaev, N., et al. (2008). Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. Journal of Cellular and Molecular Medicine, 12, 1622–1631.
Watson, N., Divers, R., Kedar, R., Mehindru, A., Mehindru, A., Borlongan, M. C., & Borlongan, C. V. (2015). Discarded Wharton’s jelly of the human umbilical cord: a viable source for mesenchymal stem cells. Cytotherapy, 17, 18–24.
Acknowledgements
We thank Lic. Lidia M. Lopez for her expert technical assistance with electron microscopy studies; Dr. Ines Rebagliata for her technical support in cell cultures and Dr. Cecilia Poderoso for kindly providing the pSUPER-retro plasmid.
Grant Support
UBACYT 20020130100258BA (to A. Brusco) and PICT 2010–2430 (to J.J. Poderoso).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors indicate no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Sanchez, V., Villalba, N., Fiore, L. et al. Characterization of Tunneling Nanotubes in Wharton’s jelly Mesenchymal Stem Cells. An Intercellular Exchange of Components between Neighboring Cells. Stem Cell Rev and Rep 13, 491–498 (2017). https://doi.org/10.1007/s12015-017-9730-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-017-9730-8