Abstract
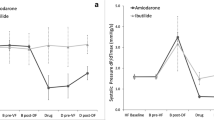
Recent experimental studies showed a protective effect of the renin inhibitor aliskiren regarding atrial structural remodeling. Purpose of this study was to assess acute electrophysiologic effects of aliskiren in a whole-heart model of atrial fibrillation (AF) and to investigate its impact on the ventricle. Twelve rabbit hearts were excised, retrogradely perfused, and paced at different cycle lengths. To enhance atrial vulnerability, a combination of acetylcholine (ACh) and isoproterenol (Iso) was infused and significantly reduced atrial action potential duration (aAPD90) and atrial effective refractory period (aERP). Additional infusion of aliskiren prolonged aAPD90 but did not alter aERP. A triangulation of action potential with ACh/Iso and a further triangulation after treatment with aliskiren were noted. Vulnerability to AF was tested by employing trains of burst pacing. Administration of ACh/Iso provoked more episodes of AF (baseline: 26 episodes, Iso/Ach: 48 episodes). Additional treatment with aliskiren induced AF significantly more often (108 episodes). Another nine hearts were perfused with aliskiren to examine its ventricular effects. Infusion with aliskiren abbreviated ventricular APD90 and ERP. Utilizing programmed ventricular stimulation, a trend towards more ventricular arrhythmias in aliskiren-treated hearts was observed. Though aliskiren did not reduce aAPD90 or aERP, acute treatment with aliskiren promoted AF. Triangulation of atrial action potentials, which is an established risk factor for ventricular proarrhythmia, may contribute to the increased atrial vulnerability. This effect may interfere with its recently demonstrated beneficial properties in atrial remodeling. Of note, aliskiren might have a proarrhythmic effect on the ventricular level.






Similar content being viewed by others
References
Kirchhof, P., Benussi, S., Kotecha, D., Ahlsson, A., Atar, D., Casadei, B., et al. (2016). 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace,18, 1609–1678.
Musini, V. M., Lawrence, K. A., Fortin, P. M., Bassett, K., & Wright, J. M. (2017). Blood pressure lowering efficacy of renin inhibitors for primary hypertension. Cochrane Database Systematic Review,4, CD007066.
Satoh, A., Niwano, S., Niwano, H., Kishihara, J., Aoyama, Y., Oikawa, J., et al. (2017). Aliskiren suppresses atrial electrical and structural remodeling in a canine model of atrial fibrillation. Heart and Vessels,32, 90–100.
Tsai, C.-F., Chen, Y.-C., Lin, Y.-K., Chen, S.-A., & Chen, Y.-J. (2011). Electromechanical effects of the direct renin inhibitor (aliskiren) on the pulmonary vein and atrium. Basic Research in Cardiology,106, 979–993.
Zhao, Z., Chen, Y., Li, W., Wang, X., Li, J., Yang, W., et al. (2016). Aliskiren protecting atrial structural remodeling from rapid atrial pacing in a canine model. Naunyn Schmiedebergs Arch Pharmacol,389, 863–871.
Zhao, Z., Wang, X., Li, J., Yang, W., Cheng, L., Chen, Y., et al. (2014). Protective effects of aliskiren on atrial ionic remodeling in a canine model of rapid atrial pacing. Cardiovascular Drugs and Therapy,28, 137–143.
Yamada, C., Kuwahara, K., Yamazaki, M., Nakagawa, Y., Nishikimi, T., Kinoshita, H., et al. (2015). The renin–angiotensin system promotes arrhythmogenic substrates and lethal arrhythmias in mice with non-ischaemic cardiomyopathy. Cardiovascular Research,109, 162–173.
Jia, Y.-Y., Bao, Z.-W., Wei, M.-F., Zhu, J.-H., & Le, G. (2013). Aliskiren ameliorates sympathetic nerve sprouting and suppresses the inducibility of ventricular tachyarrhythmia in postinfarcted rat heart. Chinese Medical Journal (England),126, 4707–4714.
Frommeyer, G., Kohnke, A., Ellermann, C., Dechering, D. G., Kochhäuser, S., Reinke, F., et al. (2017). Acute infusion of levosimendan enhances atrial fibrillation in an experimental whole-heart model. International Journal of Cardiology,236, 423–426.
Ellermann, C., Wolfes, J., Puckhaber, D., Bögeholz, N., Leitz, P., Lange, P. S., et al. (2018). Digitalis promotes ventricular arrhythmias in flecainide- and ranolazine-pretreated hearts. Cardiovascular Toxicology. https://doi.org/10.1007/s12012-018-9494-7.
Milberg, P., Ramtin, S., Mönnig, G., Osada, N., Wasmer, K., Breithardt, G., et al. (2004). Comparison of the in vitro electrophysiologic and proarrhythmic effects of amiodarone and sotalol in a rabbit model of acute atrioventricular block. Journal of Cardiovascular Pharmacology,44, 278–286.
Frommeyer, G., Milberg, P., Uphaus, T., Kaiser, D., Kaese, S., Breithardt, G., et al. (2013). Antiarrhythmic effect of ranolazine in combination with class III drugs in an experimental whole-heart model of atrial fibrillation. Cardiovascular Therapeutics,31, e63–e71.
Nattel, S., Burstein, B., & Dobrev, D. (2008). Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circulation: Arrhythmia and Electrophysiology,1, 62–73.
Hondeghem, L., Carlsson, L., & Duker, G. (2001). Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation,103, 2004–2013.
Milberg, P., Hilker, E., Ramtin, S., Cakir, Y., Stypmann, J., Engelen, M. A., et al. (2007). Proarrhythmia as a class effect of quinolones: Increased dispersion of repolarization and triangulation of action potential predict torsades de pointes. Journal of Cardiovascular Electrophysiology,18, 647–654.
Dobrev, D., & Ravens, U. (2003). Remodeling of cardiomyocyte ion channels in human atrial fibrillation. Basic Research in Cardiology,98, 137–148.
Wettwer, E., Hála, O., Christ, T., Heubach, F., Jr., Dobrev, D., Knaut, M., et al. (2004). Role of I Kur in controlling action potential shape and contractility in the human atrium: Influence of chronic atrial fibrillation. Circulation,110, 2299–2306.
Frommeyer, G., Kohnke, A., Ellermann, C., Dechering, D. G., Kochhäuser, S., Pott, C., et al. (2017). Experimental evidence for a severe proarrhythmic potential of levosimendan. International Journal of Cardiology,228, 583–587.
Vaidyanathan, S., Jarugula, V., Dieterich, H. A., Howard, D., & Dole, W. P. (2008). Clinical pharmacokinetics and pharmacodynamics of aliskiren. Clinical Pharmacokinetics,47, 515–531.
Vaidyanathan, S., Jermany, J., Yeh, C., Bizot, M. N., & Camisasca, R. (2006). Aliskiren, a novel orally effective renin inhibitor, exhibits similar pharmacokinetics and pharmacodynamics in Japanese and Caucasian subjects. British Journal of Clinical Pharmacology,62, 690–698.
Vaidyanathan, S., Camenisch, G., Schuetz, H., Reynolds, C., Yeh, C. M., Bizot, M. N., et al. (2008). Pharmacokinetics of the oral direct renin inhibitor aliskiren in combination with digoxin, atorvastatin, and ketoconazole in healthy subjects: The role of P-glycoprotein in the disposition of aliskiren. Journal of Clinical Pharmacology,48, 1323–1338.
Acknowledgements
This study was supported by the Hans-and-Gertie Fischer Foundation and by the German Cardiac Society (to G.F.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Ethical Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
Additional information
Handling Editor: Bérengère Dumotier
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ellermann, C., Mittelstedt, A., Wolfes, J. et al. Action Potential Triangulation Explains Acute Proarrhythmic Effect of Aliskiren in a Whole-Heart Model of Atrial Fibrillation. Cardiovasc Toxicol 20, 49–57 (2020). https://doi.org/10.1007/s12012-019-09533-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-019-09533-w